Chemistry:Lanthionine
| |
| Names | |
|---|---|
| IUPAC name
S-[(2R)-2-Amino-2-carboxyethyl]-L-cysteine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12N2O4S | |
| Molar mass | 208.2318 g/mol |
| Melting point | 280 to 283 °C (536 to 541 °F; 553 to 556 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
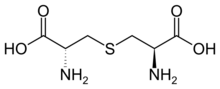
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum.
Background
In 1941, lanthionine was first isolated by treating wool with sodium carbonate. It was found to be a sulfur-containing amino acid; accordingly it was given the name lanthionine [wool (Latin: Lana), sulfur (Greek: theîon)].[1] Lanthionine was first synthesized by alkylation of cysteine with β-chloroalanine.[2] Lanthionines are found widely in nature. They have been isolated from human hair, lactalbumin, and feathers. Lanthionines have also been found in bacterial cell walls and are the components of a group of gene-encoded peptide antibiotics called lantibiotics, which includes nisin (a food preservative), subtilin, epidermin (effective against Staphylococcus and Streptococcus), and ancovenin (an enzyme inhibitor).[3][4]
Preparation
A variety of syntheses of lanthionine have been published including sulfur extrusion from cystine,[5] ring opening of serine β-lactone,[4] and hetero-conjugate addition of cysteine to dehydroalanine.[6] The sulfur extrusion method is, however, the only pathway for lanthionine that has been employed in the total synthesis of a lantibiotic.
Biosynthesis of the lanthionine bridge in peptidic natural products can be accomplished through a number of different pathways. For example, the lanthionine bridges in the antibiotic nisin are the result of a dedicated dehydratase (NisB) and a dedicated cyclase (NisC).[7][8]
References
- ↑ Horn, M. J.; Jones, D. B.; Ringel, S. J. (1941) Isolation of a New Sulfur-Containing Amino Acid (Lanthionine) from Sodium Carbonate-Treated Wool. Journal of Biological Chemistry, 138, 141-149.
- ↑ Brown, G. B.; du Vigneaud, V. (1941) The Stereoisomeric Forms of Lanthionine. Journal of Biological Chemistry, 140, 767-771.
- ↑ Paul, M.; van der Donk, W. A. (2005) Chemical and Enzymatic Synthesis of Lanthionines. Mini-Reviews in Organic Chemistry, 2, 23-37.
- ↑ 4.0 4.1 Shao, H.; Wang, S. H. H.; Lee, C.-W.; Ösapay, G.; Goodman, M. (1995) A Facile Synthesis of Orthogonally Protected Stereoisomeric Lanthionines by Regioselective Ring Opening of Serine β-Lactone Derivatives. Journal of Organic Chemistry, 60, 2956-2957.
- ↑ Harpp, D. N.; Gleason, J. G. (1971) Preparation and Mass Spectral Properties of Cystine and Lanthionine Derivatives. Novel Synthesis of L-Lanthionine by Selective Desulfurization. Journal of Organic Chemistry, 36, 73-80.
- ↑ Probert, J. M.; Rennex, D.; Bradley, M. (1996) Lanthionines for Solid Phase Synthesis. Tetrahedron Letters, 37, 1101-1104.
- ↑ "Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature". Nat Prod Rep 30 (1): 108–60. 2013. doi:10.1039/c2np20085f. PMID 23165928.
- ↑ "Discovery, biosynthesis, and engineering of lantipeptides". Annu. Rev. Biochem. 81: 479–505. 2012. doi:10.1146/annurev-biochem-060110-113521. PMID 22404629.
 |
