Chemistry:Moroidin
| |
| Names | |
|---|---|
| IUPAC name
(8S,9S,12S,15S,18S,21S,27S)-21-[3-(diaminomethylideneamino)propyl]-12-(2-methylpropyl)-10,13,16,19,22,25-hexaoxo-9-{[(2S)-5-oxopyrrolidine-2-carbonyl]amino}-8,15-di(propan-2-yl)-2,11,14,17,20,23,26,30,32-nonazapentacyclo[16.14.2.13,7.129,32.04,33]hexatriaconta-1(33),3,5,7(36),29(35),30-hexaene-27-carboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C47H66N14O10 | |
| Molar mass | 987.133 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
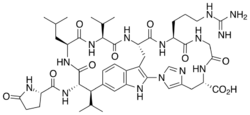
Moroidin is a biologically active compound found in the plants Dendrocnide moroides and Celosia argentea.[1] It is a peptide composed of eight amino acids, with unusual leucine-tryptophan and tryptophan-histidine cross-links that form its two rings. Moroidin has been shown to be at least one of several bioactive compounds responsible for the painful sting of the Dendrocnide moroides plant. It also has demonstrated anti-mitotic properties, specifically by inhibition of tubulin polymerization. Anti-mitotic activity gives moroidin potential as a chemotherapy drug, and this property combined with its unusual chemical structure has made it a target for organic synthesis.
Structure
Moroidin, a bicyclic octapeptide, has been isolated from Dendrocnide moroides (also called Laportea moroides) and Celosia argentea. The structure of moroidin was confirmed in 2004 by X-ray crystallography.[2] It contains two unusual crosslinks, one between leucine and tryptophan and the other between tryptophan and histidine. These linkages are also present in an analogous family of compounds, the celogentins.[3]
Total synthesis
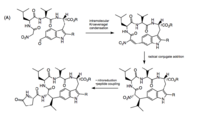
The total synthesis of moroidin has not yet been described.[4] Partial syntheses including the Leu-Trp and Trp-His linkages have been achieved. In their total synthesis of celogentin C, Castle and coworkers first obtained the Leu-Trp cross-link. The formation of this bond involved an intermolecular Knoevenagel condensation followed by radical conjugate addition and nitro reduction. This gave a product mixture of diastereomers, with the major product having the desired configuration.[3]
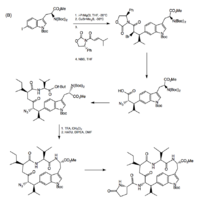
A second approach by Jia and coworkers employed an asymmetric Michael addition and bromination, a stereoselective reaction that gave a compound with the correct configuration and Leu-Trp linkage.[5]
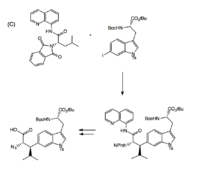
Chen and coworkers demonstrated another stereoselective approach, which coupled iodotryptophan to 8-aminoquinoline by palladium catalysis to give a single diastereomer with the desired Leu-Trp linkage and configuration.[6]
The Trp-His cross-link is addressed by Castle and coworkers, who used oxidative coupling by NCS to form the C-N linkage. To prevent over-chlorination, NCS was incubated with Pro-OBn, which reacts with NCS so as to modulate its concentration.[3] This method of cross-linking tryptophan and histidine was used in subsequent total synthesis efforts.[6]

Toxicity
Stinging toxin
Moroidin is one of several biologically active compounds isolated from the venom of Dendrocnide moroides, a member of the stinging nettle family. The plant stores its venom in silica hairs that break off when touched, delivering the toxins through the skin and inducing extreme pain.[7] Moroidin also produces a similar pain response when injected subdermally, so it is thought to be partially responsible for the plant’s toxicity. However, moroidin injections are not as potent as injections of crude matter isolated from Dendrocnide moroides, suggesting that there are additional stinging toxins in the venom.[8]
Anti-mitotic agent
Moroidin has shown to have anti-mitotic properties, chiefly by inhibiting the polymerization of tubulin.[9] Tubulin protein polymers are the major component of microtubules. During mitosis, microtubules form the organizing structure called the mitotic apparatus, which captures, aligns, and separates chromosomes. The proper alignment and separation of chromosomes is critical to ensure that cells divide their genetic material equally between daughter cells.[10] Failure to attach chromosomes to the mitotic apparatus activates the mitotic checkpoint, preventing cells from entering anaphase to proceed with cell division. Agents that disrupt microtubules therefore inhibit mitosis through activation of this checkpoint.[11]
Moroidin and its related compounds, the celogentins, inhibit tubulin polymerization. Of this family, celogentin C is the most potent (IC50 0.8×10−6 M), and it is more potent than the anti-mitotic agent vinblastine (IC50 3.0×10−6). Moroidin has the same potency as vinblastine.[12] Because of this biological activity, compounds in this family have potential as anti-cancer agents.[3]
The mechanism of tubulin disruption is not known, but the degree of biological activity has been linked to the structure of the right-hand ring containing the Trp-His linkage. Moroidin and the celogentins can be divided into three groups according to structural similarity of the right-hand ring. Celogentin C, the most potent compound, has a unique right-hand ring containing a proline residue. Moroidin and its analogous celogentins all have activity comparable to that of vinblastine, and a third group of celogentins all have reduced activity.[3] In contrast, stephanotic acid, a cyclic compound analogous only to the left-hand ring and containing the same Leu-His linkage, has no anti-mitotic activity.[12]
Other anti-tubulin agents used as chemotherapy agents have painful side effects known as neuropathy when the drugs are exposed to tissue. Although the exact mechanism for the cause of neuropathy is unknown, it is thought to be related to the degradation of microtubules, which are essential components of neurons.[13]
References
- ↑ Morita, Hiroshi; Shimbo, Kazutaka; Shigemori, Hideyuki; Kobayashi, Jun'ichi (March 2000). "Antimitotic activity of moroidin, a bicyclic peptide from the seeds of Celosia argentea". Bioorganic & Medicinal Chemistry Letters 10 (5): 469–471. doi:10.1016/S0960-894X(00)00029-9. PMID 10743950.
- ↑ Suzuki, Hayato; Morita, Hiroshi; Shiro, Motoo; Kobayashi, Jun'ichi (2004). "Celogentin K, a new cyclic peptide from the seeds of Celosia argentea and X-ray structure of moroidin". Tetrahedron 60 (11): 2489–2495. doi:10.1016/j.tet.2004.01.053.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Ma, Bing; Banerjee, Biplab; Litvinov, Dmitry N; He, Liwen; Castle, Steven L (2010). "Total synthesis of the antimitotic bicyclic peptide celogentin C". J Am Chem Soc 132 (3): 1159–1171. doi:10.1021/ja909870g. PMID 20038144.
- ↑ Li, Lei; Hu, Weimin; Jia, Yanxing (2014). "Synthetic studies of cyclic peptides stephanotic acid methyl ester, celogentin C, and moroidin". Tetrahedron 70 (42): 7753–7762. doi:10.1016/j.tet.2014.05.082.
- ↑ 5.0 5.1 Hu, Weimin; Zhang, Fengying; Xu, Zhengren; Liu, Qiang; Cui, Yuxin; Jia, Yanxing (2010). "Stereocontrolled and efficient total synthesis of (–)-stephanotic acid methyl ester and (–)-celogentin C". Org Lett 12 (5): 956–959. doi:10.1021/ol902944f. PMID 20108939.
- ↑ 6.0 6.1 6.2 6.3 Arndt H-D; Milroy L-G; Rizzo S (2011). "Biomimetic synthesis of indole-oxidized and complex peptide alkaloids". in Poupon, Edwin; Nay, Bastien. Biomimetic Organic Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA. pp. 357–394. ISBN 9783527325801. https://archive.org/details/biomimeticorgani00poup.
- ↑ Hurley, Marina (2000). "Selective stingers". ECOS. https://www.thefreelibrary.com/Selective+stingers%3A+the+stinging+tree+can+kill+dogs+and+horses,+and...-a081137827.
- ↑ Leung, T-W Christina; Williams, Dudley H; Barna, Jennifer CJ; Foti, Salvatore; Oelrichs, Peter B (1986). "Structural studies on the peptide moroidin from Laportea moroides". Tetrahedron 42 (12): 3333–3348. doi:10.1016/s0040-4020(01)87397-x.
- ↑ Morita, Hiroshi; Shimbo, Kazutaka; Shigemori, Hideyuki; Kobayashi, Jun'ichi (2000). "Antimitotic activity of moroidin, a bicyclic peptide from the seeds of Celosia argentea". Bioorg Chem Med Lett 10 (5): 469–471. doi:10.1016/s0960-894x(00)00029-9. PMID 10743950.
- ↑ Lodish, H; Berk, A; Zipursky, SL (2000). "Microtubule dynamics and motor proteins during mitosis". Molecular Cell Biology (4th ed.). New York: WH Freeman. ISBN 9780716731368. https://archive.org/details/molecularcellbio00lodi.
- ↑ Blajeski, April L; Phan, Vy A; Kottke, Timothy J; Kaufmann, Scott H (2002). "G1 and G2 cell cycle arrest following microtubule depolymerization in human breast cancer cells". J Clin Invest 110 (1): 91–99. doi:10.1172/JCI200213275. PMID 12093892.
- ↑ 12.0 12.1 Yuen, Alexander KL; Jolliffe, Katrina A; Hutton, Craig A (2006). "Preparation of the central tryptophan moiety of the celogentin/moroidin family of anti-mitotic cyclic peptides". Aust J Chem 59 (11): 819–826. doi:10.1071/CH06324.
- ↑ Mukhtar, Eiman; Adhami, Vaqar M; Mukhtar, Hasan (2014). "Targeting microtubules by natural agents for cancer therapy". Mol Cancer Ther 13 (2): 275–284. doi:10.1158/1535-7163.MCT-13-0791. PMID 24435445.
 |