Chemistry:3-Dehydroquinic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
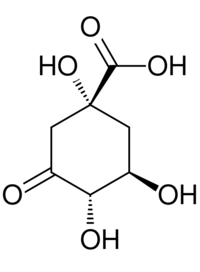
(1R,3R,4S)-1,3,4-Trihydroxy-5-oxocyclohexane-1-carboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H10O6 | |
| Molar mass | 190.152 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Dehydroquinic acid (DHQ) is the first carbocyclic intermediate of the shikimate pathway.[1] It is created from 3-deoxyarabinoheptulosonate 7-phosphate, a 7-carbon ulonic acid, by the enzyme DHQ synthase. The mechanism of ring closure is complex, but involves an aldol condensation at C-2 and C-7.
It has the same structure as quinic acid, which is found in coffee, but the C-3 hydroxyl is oxidized to a ketone group. 3-Dehydroquinic acid undergoes five further enzymatic steps in the remainder of the shikimate pathway to chorismic acid, a precursor to tyrosine, 3-phenylalanine, tryptophan, and some vitamins, including:
- Vitamin K
- Pteroylmonoglutamic acid, called folate.
3-Dehydroquinate can also be a precursor to pyrroloquinoline quinone (PQQ), an alternate redox coenzyme involved in oxidative phosphorylation.
Biosynthesis
3-Dehydroquinate goes through beta oxidation, similar to fatty acids. Then, this compound (6-oxo-3-dehydro-quinate) is transaminated to 6-amino-3-dehydroquinate. Then 6-amino-3-dehydro-quinate is dehydrated and reduced to 6-amino-4-desoxy-3-keto-quinate, which reacts with dehydroalanine and alpha-ketoglutarate, to form hexahydro-pyrroloquinoline quinone.[2] This compound is oxidized by FAD to PQQ.
References
- ↑ Morrow, Gary W. (2016). "The Shikimate Pathway: Biosynthesis of Phenolic Products from Shikimic Acid". doi:10.1093/oso/9780199860531.001.0001. https://academic.oup.com/pages/op-migration-welcome.
- ↑ Peón, Antonio; Otero, José M.; Tizón, Lorena; Prazeres, Verónica F. V.; Llamas-Saiz, Antonio L.; Fox, Gavin C.; van Raaij, Mark J.; Lamb, Heather et al. (2010-09-02). "Understanding the Key Factors that Control the Inhibition of Type II Dehydroquinase by (2R)-2-Benzyl-3-dehydroquinic Acids" (in en). ChemMedChem 5 (10): 1726–1733. doi:10.1002/cmdc.201000281. PMID 20815012. https://onlinelibrary.wiley.com/doi/10.1002/cmdc.201000281.
 |

