Chemistry:Pyrroloquinoline quinone
| |
| Names | |
|---|---|
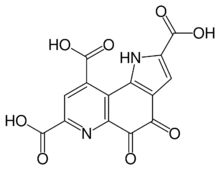
| Systematic IUPAC name
4,5-Dioxo-4,5-dihydro-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 3596812 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| 56633 | |
| KEGG | |
| MeSH | PQQ+Cofactor |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H6N2O8 | |
| Molar mass | 330.208 g·mol−1 |
| Density | 1.963 g/cm3 |
| Hazards | |
| Flash point | 569.8 °C (1,057.6 °F; 842.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pyrroloquinoline quinone (PQQ), also called methoxatin, is a redox cofactor and antioxidant.[1]
Quinoprotein glucose dehydrogenase is used as a glucose sensor in bacteria. PQQ stimulates growth in bacteria.[2]
History
It was discovered by J. G. Hauge as the third redox cofactor after nicotinamide and flavin in bacteria (although he hypothesised that it was naphthoquinone).[3] Anthony and Zatman also found the unknown redox cofactor in alcohol dehydrogenase. In 1979, Salisbury and colleagues[4] as well as Duine and colleagues[5] extracted this prosthetic group from methanol dehydrogenase of methylotrophs and identified its molecular structure. Adachi and colleagues discovered that PQQ was also found in Acetobacter.[6]
Biosynthesis
A novel aspect of PQQ is its biosynthesis in bacteria from a ribosomally translated precursor peptide, PqqA (UniProt P27532).[7] A glutamic acid and a tyrosine in PqqA are cross-linked by the radical SAM enzyme PqqE (P07782) with the help of PqqD (P07781) in the first step of PqqA modification.[8] A protease then liberates the Glu-Tyr molecule from the peptide backbone. PqqB (P07779) oxidizes the 2 and 3 positions on the tyrosine ring, forming a quinone which quickly becomes AHQQ, finishing the pyridine ring. PqqC (P07780) then forms the final pyrrole ring.[9]
Efforts to understand PQQ biosynthesis have contributed to broad interest in radical SAM enzymes and their ability to modify proteins, and an analogous radical SAM enzyme-dependent pathway has since been found that produces the putative electron carrier mycofactocin, using a valine and a tyrosine from the precursor peptide, MftA (P9WJ81).[8]
Role in proteins
Quinoproteins generally embed the cofactor in a unique, six-bladed[10] beta-barrel structure. Some examples also have a heme c prosthetic group and are termed quinohemoproteins.[11] Although quinoproteins are mostly found in bacteria, a Coprinopsis cinerea (fungus) pyranose dehydrogenase has been shown to use PQQ in its crystal structure.[10]
PQQ also appears to be essential in some other eukaryotic proteins, albeit not as the direct electron carrier. The aforementioned mammalian lactate dehydrogenase requires PQQ to run but uses NADH as the direct redox cofactor. It seems to speed up the reaction by catalyzing the oxidation of NADH via redox cycling.[12]
Controversy regarding role as vitamin
The scientific journal Nature published the 2003 paper by Kasahara and Kato that essentially stated that PQQ was a new vitamin and in 2005, an article by Anthony and Felton that stated that the 2003 Kasahara and Kato paper drew incorrect and unsubstantiated conclusions.[13] An article by Bruce Ames in The Proceedings of the National Academy of Sciences in 2018 identified pyrroloquinoline quinone as a "longevity vitamin" not essential for immediate survival, but necessary for long-term health.[14]
See also
References
- ↑ Wen H, He Y, Zhang K, Yang X, Hao D, Jiang Y, He B. Mini-review: Functions and Action Mechanisms of PQQ in Osteoporosis and Neuro Injury. Curr Stem Cell Res Ther. 2020;15(1):32-36. doi:10.2174/1574888X14666181210165539 PMID 30526470
- ↑ "Pyrroloquinoline quinone: excretion by methylotrophs and growth stimulation for microorganisms". BioFactors 1 (1): 51–3. 1988. PMID 2855583.
- ↑ Hauge JG (1964). "Glucose dehydrogenase of bacterium anitratum: an enzyme with a novel prosthetic group". J Biol Chem 239 (11): 3630–9. doi:10.1016/S0021-9258(18)91183-X. PMID 14257587.
- ↑ "A novel coenzyme from bacterial primary alcohol dehydrogenases". Nature 280 (5725): 843–4. 1979. doi:10.1038/280843a0. PMID 471057. Bibcode: 1979Natur.280..843S.
- ↑ "The prosthetic group of methanol dehydrogenase from Hyphomicrobium X: electron spin resonance evidence for a quinone structure". Biochem Biophys Res Commun 87 (3): 719–24. 1979. doi:10.1016/0006-291X(79)92018-7. PMID 222269.
- ↑ "Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria". FEBS Lett 130 (2): 179–83. 1981. doi:10.1016/0014-5793(81)81114-3. PMID 6793395.
- ↑ "A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone.". J Bacteriol 174 (4): 1426–7. 1992. doi:10.1128/jb.174.4.1426-1427.1992. PMID 1310505.
- ↑ 8.0 8.1 Haft DH (2011). "Bioinformatic evidence for a widely distributed, ribosomally produced electron carrier precursor, its maturation proteins, and its nicotinoprotein redox partners.". BMC Genomics 12: 21. doi:10.1186/1471-2164-12-21. PMID 21223593.
- ↑ Zhu, W; Klinman, JP (December 2020). "Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone.". Current Opinion in Chemical Biology 59: 93–103. doi:10.1016/j.cbpa.2020.05.001. PMID 32731194.
- ↑ 10.0 10.1 Takeda, K; Ishida, T; Yoshida, M; Samejima, M; Ohno, H; Igarashi, K; Nakamura, N (15 December 2019). "Crystal Structure of the Catalytic and Cytochrome b Domains in a Eukaryotic Pyrroloquinoline Quinone-Dependent Dehydrogenase.". Applied and Environmental Microbiology 85 (24). doi:10.1128/AEM.01692-19. PMID 31604769. Bibcode: 2019ApEnM..85E1692T.
- ↑ Matsushita, K; Toyama, H; Yamada, M; Adachi, O (January 2002). "Quinoproteins: structure, function, and biotechnological applications.". Applied Microbiology and Biotechnology 58 (1): 13–22. doi:10.1007/s00253-001-0851-1. PMID 11831471.
- ↑ Akagawa, M; Minematsu, K; Shibata, T; Kondo, T; Ishii, T; Uchida, K (27 May 2016). "Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein.". Scientific Reports 6: 26723. doi:10.1038/srep26723. PMID 27230956. Bibcode: 2016NatSR...626723A.
- ↑ "Biochemistry: role of PQQ as a mammalian enzyme cofactor?". Nature 433 (7025): E10; discussion E11–2. 2005. doi:10.1038/nature03322. PMID 15689995. Bibcode: 2005Natur.433E..10F.
- ↑ Ames, Bruce (15 October 2018). "Prolonging healthy aging: Longevity vitamins and proteins". Proceedings of the National Academy of Sciences of the United States of America 115 (43): 10836–10844. doi:10.1073/pnas.1809045115. PMID 30322941. Bibcode: 2018PNAS..11510836A.
- ↑ "L-aminoadipate-semialdehyde dehydrogenase (Homo sapiens)". L-aminoadipate-semialdehyde dehydrogenase (Homo sapiens). Technische Universität Braunschweig. July 2015. http://www.brenda-enzymes.org/enzyme.php?ecno=1.2.1.31&Suchword=&organism%5B%5D=Homo+sapiens&show_tm=0. Retrieved 18 July 2015.
- ↑ "Pyrroloquinoline quinone (HMDB13636)". University of Alberta. http://www.hmdb.ca/metabolites/HMDB13636. "Enzymes containing PQQ are called quinoproteins. PQQ and quinoproteins play a role in the redox metabolism and structural integrity of cells and tissues PMID 2558842. It was reported that aminoadipate semialdehyde dehydrogenase (AASDH) might also use PQQ as a cofactor, suggesting a possibility that PQQ is a vitamin in mammals. PMID 12712191."
 |

