Biology:Anopheles
| Anopheles | |
|---|---|
| |
| Anopheles stephensi, female | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Family: | Culicidae |
| Subfamily: | Anophelinae |
| Genus: | Anopheles Meigen 1818 |
| Species | |
|
For a full description, see the main article: Taxonomy of Anopheles | |
Anopheles (/əˈnɒfɪliːz/) is a genus of mosquito first described by J. W. Meigen in 1818. Its members are sometimes called nail mosquitoes or marsh mosquitoes.[1] Many are vectors of the parasite Plasmodium, which causes malaria in birds, reptiles, and mammals including humans. Anopheles gambiae is the best-known species, as it transmits one of the most dangerous human malarial parasites, Plasmodium falciparum. No other mosquito genus is a vector of human malaria.
The genus diverged from culicine mosquitoes at least 100 million years ago (mya). Like other mosquitoes, its eggs, larvae, and pupae are aquatic. The larva has no respiratory siphon to breathe through, so it feeds with its body horizontal at the water surface. The adult hatches from the surface and feeds on nectar; females also feed on blood, allowing them to carry parasites between hosts. The adult's feeding position is head-down, unlike the horizontal stance of culicines. Anopheles species are distributed nearly worldwide across the tropics, subtropics, and temperate regions. Adults aestivate in hot dry weather, enabling them to survive in hot dry regions like the Sahel.
Evolution
Fossil history
Fossils of this genus are rare; only two had been found by 2015.[2] They are Anopheles (Nyssorhynchus) dominicanus Zavortink & Poinar in Dominican amber from the Late Eocene (40.4 million years ago to 33.9 million years ago),[3] and Anopheles rottensis Statz in Germany amber from the Late Oligocene (28.4 million years ago to 23 million years ago).[2]
Phylogeny
The ancestors of all flies including mosquitoes appeared 260 million years ago.[4] The culicine and Anopheles clades of mosquitoes diverged between 120 million years ago and 150 million years ago.[4][5] The Old and New World Anopheles species subsequently diverged between 80 million years ago and 95 million years ago.[4][5] Anopheles darlingi diverged from the African and Asian malaria vectors ~100 million years ago.[6] The cladogram is based on an analysis of mosquito genomes by Heafsey and colleagues in 2015:[6]
| Diptera |
| ||||||||||||||||||||||||||||||||||||||||||
| 260 mya |
Taxonomy
The genus Anopheles was introduced by the German entomologist Johann Wilhelm Meigen in 1818. He described two species, A. birfurcatus and the type species, Anopheles maculipennis. He stated that the name of the genus meant beschwerlich, "burdensome".[7] The name comes from the Ancient Greek word ἀνωφελής anōphelḗs 'useless', derived from ἀν- an-, 'not', 'un-' and ὄφελος óphelos 'profit'.[8]
The taxonomy of the genus was greatly advanced in 1901 when the English entomologist Frederick Vincent Theobald described 39 Anopheles species in his 5-volume monograph on the Culicidae.[9] He was provided with mosquito specimens sent in to the British Museum (Natural History) from around the world, on the 1898 instruction of the Secretary of State for the Colonies, Joseph Chamberlain.[10]
Anopheles (with a nearly worldwide distribution) belongs to the subfamily Anophelinae alongside two other genera: Bironella (restricted to Australia ) and Chagasia (restricted to the Neotropics). The taxonomy remains incompletely settled.[11][12] Classification into species is based on morphological characteristics – wing spots, head anatomy, larval and pupal anatomy, chromosome structure, and more recently, on DNA sequences.[13][14][15] In the taxonomy published by Harbach and Kitching in 2016, it was shown that three species of Bironella (B. confusa, B. gracilis, and B. hollandi) are phylogenetically more similar to A. kyondawensis than other Bironella species. That phylogeny argues that, based on genetic similarity, A. implexus is divergent from the common ancestor to the Anopheles genus.[10]
Life cycle
Like all mosquitoes, anophelines go through four stages in their life cycles: egg, larva, pupa, and adult. The first three stages are aquatic and together last 5–14 days, depending on the species and the ambient temperature. The adult stage is when the female Anopheles mosquito acts as malaria vector. The adult females can live up to a month (or more in captivity), but most probably do not live more than two weeks in nature.[16]
Eggs

Adult females lay 50–200 eggs per oviposition. The eggs are quite small (about 0.5 millimetres (0.02 in) × 0.2 millimetres (0.008 in)). Eggs are laid singly and directly on water. They are unique in that they have floats on either side. Eggs are not resistant to drying and hatch within 2–3 days, although hatching may take up to 2–3 weeks in colder climates.[16]
Larvae
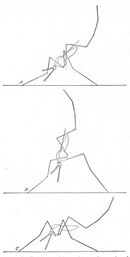
The mosquito larva has a well-developed head with mouth brushes used for feeding, a large thorax and a nine-segment abdomen. It has no legs. In contrast to other mosquitoes, the Anopheles larva lacks a respiratory siphon, so it positions itself so that its body is parallel to the surface of the water. In contrast, the feeding larva of culicine mosquitoes attach themselves to the water surface with the posterior siphon, the body pointing downwards. Larvae breathe through spiracles located on the eighth abdominal segment and so must come to the surface frequently. The larvae spend most of their time feeding on algae, bacteria, and other microorganisms in the thin surface layer. They dive below the surface only when disturbed. Larvae swim either by jerky movements of the entire body or through propulsion with the mouth brushes.[16]
Larvae develop through four stages, or instars, after which they metamorphose into pupae. At the end of each instar, the larvae molt, shedding their exoskeletons, or skin, to allow for further growth. The larvae occur in a wide range of habitats, but most species prefer clean, unpolluted water. Larvae of Anopheles mosquitoes have been found in freshwater or saltwater marshes, mangrove swamps, rice fields, grassy ditches, the edges of streams and rivers, and small, temporary rain pools. Many species prefer habitats with vegetation. Others prefer habitats with none. Some breed in open, sun-lit pools, while others are found only in shaded breeding sites in forests. A few species breed in tree holes or the leaf axils of some plants.[16]
-
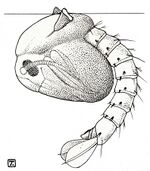
Anopheles larva
-
Feeding position of an Anopheles larva (A), culicine larva with its siphon (B)
Pupae
The pupa (also known as a tumbler) is comma-shaped when viewed from the side. The head and thorax are merged into a cephalothorax, with the abdomen curving around underneath it. As with the larvae, the pupa must come to the surface frequently to breathe, which it does through a pair of respiratory trumpets on its cephalothorax. After a few days as a pupa, the dorsal surface of the cephalothorax splits and the adult mosquito emerges.[16]
-
A. maculipennis pupa, breathing at the surface
Adults
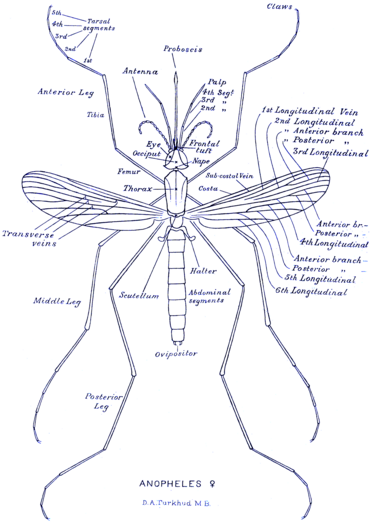
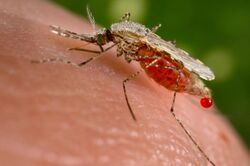
Like all mosquitoes, adult Anopheles species have slender bodies with three sections: head, thorax and abdomen. The head is specialized for acquiring sensory information and for feeding. It contains the eyes and a pair of long, many-segmented antennae. The antennae are important for detecting host odours, as well as of breeding sites where females lay eggs.[16] Female mosquitoes carrying Plasmodium parasites, the causative agents of malaria, are significantly more attracted to human breath and odours than uninfected mosquitoes.[17] The head has an elongated, forward-projecting proboscis used for feeding, and two maxillary palps. These palps carry the receptors for carbon dioxide, a major attractant that enables the mosquito to locate its host. The thorax is specialized for locomotion. Three pairs of legs and a pair of wings are attached to the thorax. The abdomen is specialized for food digestion and egg development. This segmented body part expands considerably when a female takes a blood meal. The blood is digested over time, serving as a source of protein for the production of eggs, which gradually fill the abdomen.[16]
Anopheles can be distinguished from other mosquitoes by the palps, which are as long as the proboscis, and by the presence of discrete blocks of black and white scales on the wings. Adults can further be identified by their typical resting position: both sexes rest with their abdomens pointing up, unlike culicine mosquitoes. Adult mosquitoes usually mate within a few days after emerging from the pupal stage. In most species, the males form large swarms, usually around dusk, and the females fly into the swarms to mate. The duration from egg to adult varies considerably among species, and is strongly influenced by ambient temperature. Mosquitoes can develop from egg to adult in as little as five days, but it can take 10–14 days in tropical conditions.[16]
Males live for about a week, feeding on nectar and other sources of sugar. Males cannot feed on blood, as it appears to produce toxic effects and kills them within a few days, around the same lifespan as a water-only diet.[18] Females feed on sugar sources for energy, but usually require a blood meal for the development of eggs. After obtaining a full blood meal, the female rests for a few days while the blood is digested and eggs are developed. This process depends on the temperature, but usually takes 2–3 days in tropical conditions. Once the eggs are fully developed, the female lays them and resumes host-seeking. The cycle repeats itself until the female dies. While females can live longer than a month in captivity, most do not live longer than one to two weeks in nature. Their lifespans depend on temperature, humidity, and their ability to successfully obtain a blood meal while avoiding host defenses.[16]
-
Morphology of female Anopheles
Ecology
Distribution
Anopheles species live both in tropical areas known for malaria such as sub-Saharan Africa, and in colder latitudes. Indeed, malaria outbreaks have in the past occurred in colder climates, for example during the construction of the Rideau Canal in Canada during the 1820s.[19] Anopheles species that can transmit malaria are not limited to malaria-endemic areas, so areas where they have been eliminated are constantly at risk of reintroduction of the disease.[20]

Habitat
Anopheles mosquitoes require bodies of water, possibly small and seasonal, for their aquatic larvae and pupae. Suitable habitats range from ponds to water tanks, swamps, ditches and puddles.[21] The adults can however live in dry regions such as Africa's savanna and Sahel. They can travel far from water, and are sometimes blown hundreds of kilometres by suitable winds. Adults can aestivate for months at a time, becoming dormant in hot dry weather, allowing them to persist through the African dry season.[22] Further, Anopheles mosquitoes have been documented travelling in baggage, such as on aircraft.[23]
Parasites
Parasites of Anopheles include Microsporidia of the genera Amblyospora, Crepidulospora, Senoma and Parathelohania.[24] Two distinct life cycles are found in the Microsporidia. In the first type, the parasite is transmitted by the oral route and is relatively species nonspecific. In the second, while again the oral route is the usual route of infection, the parasite is ingested within an already infected intermediate host. Infection of the insect larval form is frequently tissue-specific, and commonly involves the fat body. Vertical (transovarial) transmission also occurs.[25]
The parasitic Wolbachia bacteria have been studied for use as control agents.[26]
Predators
The jumping spider Evarcha culicivora indirectly feeds on vertebrate blood by preying on female Anopheles mosquitos.[27] Juvenile spiders choose the Anopheles over all other prey regardless of whether it actually is carrying blood.[28] Juvenile spiders have adopted an Anopheles-specific prey-capture behavior, using the posture of Anopheles mosquitoes as a primary cue to identify them.[27] Anopheles has a distinctive resting posture with its abdomen angled up. In this case, the spider approaches from behind the mosquito and under its abdomen, and then attacks from below.[29]
Malaria vectors
Preferred sources for blood meals
Since the genus Anopheles is the sole vector for malaria, it has been studied intensively in the search for effective control methods. One important behavioral factor is the degree to which an Anopheles species prefers to feed on humans (anthropophily) or animals such as cattle or birds (zoophily). Anthropophilic Anopheles are more likely to transmit the malaria parasites from one person to another. Most Anopheles mosquitoes are not exclusively anthropophilic or zoophilic, including the primary malaria vector in the western United States, A. freeborni.[30][31] However, the primary malaria vectors in Africa, A. gambiae and A. funestus, are strongly anthropophilic and are consequently major vectors of human malaria.[16]
Probability of transmitting malaria
Once ingested by a mosquito, malaria parasites must undergo development within the mosquito before they are infectious to humans. The time required for the parasite to develop in the mosquito (the extrinsic incubation period) ranges from 10–21 days, depending on the parasite species and the temperature. If a mosquito does not survive long enough for the parasite to develop, then she transmits no parasites.[16]
It is not possible to measure directly the lifespans of mosquitoes in nature, but indirect estimates of daily survivorship have been made for several Anopheles species. Estimates of daily survivorship in Tanzania of A. gambiae, the vector of the dangerous Plasmodium falciparum parasite, ranged from 0.77 to 0.84, meaning that after one day, between 77% and 84% have survived.[32] Assuming this survivorship is constant through the adult life of a mosquito, less than 10% of female A. gambiae would survive longer than a 14-day extrinsic incubation period. If daily survivorship increased to 0.9, over 20% of mosquitoes would survive longer than the same period. Control measures that rely on insecticides (e.g. indoor residual spraying) may actually impact malaria transmission more through their effect on adult longevity than through their effect on the population of adult mosquitoes.[16]
Patterns of feeding and resting
Most Anopheles mosquitoes are crepuscular (active at dusk or dawn) or nocturnal (active at night). Some feed indoors (endophagic), while others feed outdoors (exophagic). After feeding, some blood mosquitoes prefer to rest indoors (endophilic), while others prefer to rest outdoors (exophilic). Biting by nocturnal, endophagic Anopheles mosquitoes can be markedly reduced through the use of insecticide-treated bed nets or through improved housing construction to prevent mosquito entry (e.g. window screens). Endophilic mosquitoes are readily controlled by indoor spraying of residual insecticides. In contrast, exophagic/exophilic vectors are best controlled by destroying breeding sites, such as by filling in ponds.[16]
Gut flora
Because transmission of disease by the mosquito requires ingestion of blood, the gut flora may have a bearing on the success of infection of the mosquito host. The larval and pupal gut is largely colonized by photosynthetic cyanobacteria, while in the adult, gram-negative bacteria in the Pseudomonadota and Bacteroidota phyla predominate. Blood meals drastically reduce the diversity of microorganisms in the gut, favouring bacteria.[33]
Control
Insecticide control and resistance
Insecticides have offered a first line of approach to ridding areas of malarial mosquitoes. However, mosquitoes, with a short generation time, may rapidly evolve resistance, as experienced during the Global Malaria Eradication Campaign of the 1950s.[34] The use of insecticides in agriculture has resulted in resistance in mosquito populations, implying that an effective control program must monitor for resistance and switch to other means if resistance is detected.[35]
Eradication
In 2016, a CRISPR-Cas9 gene drive system was proposed to eradicate Anopheles gambiae,[36] by deleting the dsx gene, causing female sterility. Such a gene drive system has been shown to suppress an entire caged A. gambiae population[37] within 7–11 generations, typically less than a year. This has raised concerns with both the efficiency of a gene drive system as well as the ethical and ecological impact of such an eradication program.[38] Therefore, there have been efforts to use the gene drive system to more efficiently introduce genes of Plasmodium resistance into the species, such as targeting and knocking out the FREP1 gene in Anopheles gambiae.[39] Researchers in Burkina Faso have created a strain of the fungus Metarhizium pinghaense that is genetically engineered to produce the venom of an Australian funnel-web spider; exposure to the fungus caused populations of Anopheles mosquitoes to crash by 99% in a controlled trial.[40]
See also
References
- ↑ "Nail Mosquito". United States Fish and Wildlife Service. https://www.fws.gov/species/nail-mosquito-anopheles. "Common Name: Nail Mosquito, marsh mosquitoes"
- ↑ 2.0 2.1 Freitas, Lucas A.; Russo, Claudia A. M.; Voloch, Carolina M.; Mutaquiha, Olívio C. F.; Marques, Lucas P.; Schrago, Carlos G. (2015-08-05). "Diversification of the Genus Anopheles and a Neotropical Clade from the Late Cretaceous". PLOS ONE 10 (8): e0134462. doi:10.1371/journal.pone.0134462. PMID 26244561. Bibcode: 2015PLoSO..1034462F.
- ↑ Zavortink, Thomas J.; Poinar, George O. (2000-11-01). "Anopheles (Nyssorhynchus) dominicanus sp. n. (Diptera: Culicidae) from Dominican Amber". Annals of the Entomological Society of America 93 (6): 1230–1235. doi:10.1603/0013-8746(2000)093[1230:ANDSND2.0.CO;2].
- ↑ 4.0 4.1 4.2 Moreno, Marta; Marinotti, Osvaldo; Krzywinski, Jaroslaw; Tadei, Wanderli P.; James, Anthony A.; Achee, Nicole L.; Conn, Jan E. (2010). "Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time". Malaria Journal 9 (1): 127. doi:10.1186/1475-2875-9-127. PMID 20470395.
- ↑ 5.0 5.1 Calvo, Eric; Pham, Van M.; Marinotti, Osvaldo; Andersen, John F.; Ribeiro, José M. C. (2009). "The salivary gland transcriptome of the neotropical malaria vector Anopheles darlingi reveals accelerated evolution of genes relevant to hematophagy". BMC Genomics 10 (1): 57. doi:10.1186/1471-2164-10-57. PMID 19178717.
- ↑ 6.0 6.1 Neafsey, Daniel E. et al. (2015-01-02). "Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes". Science 347 (6217): 43. doi:10.1126/science.1258522. PMID 25554792. Bibcode: 2015Sci...347...43N.
- ↑ Meigen, Johann Wilhelm (1818) (in de). Systematische Beschreibung der bekannten Europäischen zweiflügeligen Insekten. 1. Aachen: Friedrich Wilhelm Forstmann. pp. 10–12. https://www.biodiversitylibrary.org/item/45833#page/54/mode/1up.
- ↑ Stevenson, Angus (19 August 2010). Oxford Dictionary of English. Oxford University Press. p. 64. ISBN 978-0-19-957112-3. https://books.google.com/books?id=anecAQAAQBAJ&pg=PA64.
- ↑ Theobald, Frederick Vincent (1901). A Monograph of the Culicidae, or Mosquitoes. 1. London: British Museum (Natural History). pp. 115–214. ISBN 978-1178519037. https://archive.org/details/amonographculic03zoolgoog/page/n135/mode/2up.
- ↑ 10.0 10.1 Harbach, R. E.; Kitching, I. (January 2016). "The phylogeny of Anophelinae revisited: inferences about the origin and classification of Anopheles (Diptera: Culicidae)". Zoologica Scripta 45: 34–47. doi:10.1111/zsc.12137. https://nhm.openrepository.com/handle/10141/612216.
- ↑ Krzywinski, Jaroslaw; Besansky, Nora J. (2003). "Molecular Systematics of Anopheles: From Subgenera to Subpopulations". Annual Review of Entomology 48: 111–139. doi:10.1146/annurev.ento.48.091801.112647. PMID 12208816.
- ↑ Foley, Desmond H.; Bryan, Joan H.; Yeates, David; Saul, Allan (1998). "Evolution and Systematics of Anopheles:Insights from a Molecular Phylogeny of Australasian Mosquitoes". Molecular Phylogenetics and Evolution 9 (2): 262–275. doi:10.1006/mpev.1997.0457. PMID 9562985.
- ↑ Rattanarithikul, R.; Harrison, B. A.; Harbach, R. E.; Panthusiri, P.; Coleman, R. E.; Panthusiri, P. (2006). "Illustrated keys to the mosquitoes of Thailand. IV. Anopheles". The Southeast Asian Journal of Tropical Medicine and Public Health 37 (Suppl 2): 1–128. PMID 17262930.
- ↑ Walton, C.; Somboon, P.; o'Loughlin, S. M.; Zhang, S.; Harbach, R.E. et al. (2007). "Genetic diversity and molecular identification of mosquito species in the Anopheles maculatus group using the ITS2 region of rDNA". Infection, Genetics and Evolution 7 (1): 93–102. doi:10.1016/j.meegid.2006.05.001. PMID 16782411.
- ↑ Garros, C.; Harbach, R. E; Manguin, S. (2005). "Morphological assessment and molecular phylogenetics of the Funestus and Minimus groups of Anopheles (Cellia)". Journal of Medical Entomology 42 (4): 522–536. doi:10.1093/jmedent/42.4.522. PMID 16119539.
- ↑ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12
 One or more of the preceding sentences incorporates text from a work now in the public domain: "Anopheles Mosquitoes". Centers for Disease Control and Prevention. October 21, 2015. https://www.cdc.gov/malaria/about/biology/mosquitoes/.
One or more of the preceding sentences incorporates text from a work now in the public domain: "Anopheles Mosquitoes". Centers for Disease Control and Prevention. October 21, 2015. https://www.cdc.gov/malaria/about/biology/mosquitoes/.
- ↑ Smallegange, Renate C.; van Gemert, Geert-Jan; van de Vegte-Bolmer, Marga; Gezan, Salvador; Takken, Willem; Sauerwein, Robert W.; Logan, James G. (2013-05-15). "Malaria Infected Mosquitoes Express Enhanced Attraction to Human Odor". PLOS ONE 8 (5): e63602. doi:10.1371/journal.pone.0063602. PMID 23691073. Bibcode: 2013PLoSO...863602S.
- ↑ Nikbakhtzadeh, Mahmood R.; Buss, Garrison K.; Leal, Walter S. (2016-01-26). "Toxic Effect of Blood Feeding in Male Mosquitoes". Frontiers in Physiology 7: 4. doi:10.3389/fphys.2016.00004. PMID 26858651.
- ↑ Wylie WNT (1983). "Poverty, Distress, and Disease: Labour and the Construction of the Rideau Canal, 1826–32". Labour/Le Travail 11: 7–29. doi:10.2307/25140199.
- ↑ 20.0 20.1 "CDC - Malaria - About Malaria - Where Malaria occurs". April 9, 2020. https://www.cdc.gov/malaria/about/distribution.html.
- ↑ "Anopheles Mosquitoes". https://globalvectorhub.tghn.org/vector-species/anopheles-mosquitoes/.
- ↑ Baldini, Francesco; Viana, Mafalda (2023). "Dried out but alive: how mosquitoes survive 8 months". Trends in Parasitology 39 (1): 1–3. doi:10.1016/j.pt.2022.11.006. PMID 36470782.
- ↑ Ibáñez-Justicia, Adolfo; Smitz, Nathalie; den Hartog, Wietse; van de Vossenberg, Bart; De Wolf, Katrien et al. (2020-05-15). "Detection of Exotic Mosquito Species (Diptera: Culicidae) at International Airports in Europe". International Journal of Environmental Research and Public Health 17 (10): 3450. doi:10.3390/ijerph17103450. PMID 32429218.
- ↑ Simakova, A. V.; Pankova, T. F. (2008). "Ecology and epizootology of microsporidia in malarial mosquitoes (Diptera: Culicidae) from the south of western Siberia" (in ru). Parazitologiia 42 (2): 139–150. PMID 18664069.
- ↑ Baker, Michael D.; Vossbrinck, Charles R.; Becnel, James J.; Andreadis, Theodore G. (1998). "Phylogeny of Amblyospora (Microsporida: Amblyosporidae) and related genera based on small subunit ribosomal DNA data: a possible example of host parasite cospeciation". Journal of Invertebrate Pathology 71 (3): 199–206. doi:10.1006/jipa.1997.4725. PMID 9538024. http://www.ct.gov/caes/LIB/caes/documents/biographies/BakerJIP98.pdf.
- ↑ "Mosquito Parasite Fights Infectious Disease". 1 October 2009. http://news.discovery.com/animals/mosquito-parasite-disease-fighting.html.
- ↑ 27.0 27.1 Nelson, Ximena J.; Jackson, Robert R.; Sune, Godfrey (2005). "Use of Anopheles-specific prey-capture behavior by the small juveniles of Evarcha culicivora, a mosquito-eating jumping spider". The Journal of Arachnology 33 (2): 541–548. doi:10.1636/05-3.1. https://doi.org/10.1636/05-3.1.
- ↑ Jackson, Robert R.; Cross, Fiona R. "Mosquito-terminator spiders and the meaning of predatory specialization." The Journal of Arachnology 43.2 (2015): 123–142.
- ↑ Nelson, Ximena J.; Jackson, Robert R. (2006). "A Predator from East Africa that Chooses Malaria Vectors as Preferred Prey". PLOS ONE 1 (1): 132. doi:10.1371/journal.pone.0000132. PMID 17205136. Bibcode: 2006PLoSO...1..132N.
- ↑ Carpenter, S. J.; LaCasse, W. J. (1955). Mosquitoes of North America (North of Mexico). Berkeley, Los Angeles, London: University of California Press. pp. 39–42. ISBN 0-520-02638-1.
- ↑ McHugh, Chad P. (1989-08-01). "Ecology of a Semi-Isolated Population of Adult Anopheles Freeborni: Abundance, Trophic Status, Parity, Survivorship, Gonotrophic Cycle Length, and Host Selection". The American Journal of Tropical Medicine and Hygiene 41 (2): 169–176. doi:10.4269/ajtmh.1989.41.169. PMID 2774063.
- ↑ Charlwood, J. D.; Smith, T.; Billingsley, P. F.; Takken, W.; Lyimo, E. O. K.; Meuwissen, J. H. E. T. (1997). "Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans". Bulletin of Entomological Research 87 (5): 445–453. doi:10.1017/S0007485300041304. http://doc.rero.ch/record/298415/files/S0007485300041304.pdf.
- ↑ Leulier, François, ed (2011). "Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya". PLOS ONE 6 (9): e24767. doi:10.1371/journal.pone.0024767. PMID 21957459. Bibcode: 2011PLoSO...624767W.
- ↑ Najera, J. A. (1999). "Malaria Control: Achievements, Problems, & Strategies". World Health Organization. https://iris.who.int/bitstream/handle/10665/66640/WHO_MAL_99.1087.pdf?sequence=1.
- ↑ "Biology: Anopheles Mosquitoes (tab 5)". Centers for Disease Control and Prevention. 16 July 2020. https://www.cdc.gov/malaria/about/biology/#tabs-1-5.
- ↑ Hammond, Andrew; Galizi, Roberto; Kyrou, Kyros; Simoni, Alekos; Siniscalchi, Carla et al. (January 2016). "A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae". Nature Biotechnology 34 (1): 78–83. doi:10.1038/nbt.3439. PMID 26641531.
- ↑ Kyrou, Kyros; Hammond, Andrew M.; Galizi, Roberto; Kranjc, Nace; Burt, Austin; Beaghton, Andrea K.; Nolan, Tony; Crisanti, Andrea (November 2018). "A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes". Nature Biotechnology 36 (11): 1062–1066. doi:10.1038/nbt.4245. PMID 30247490.
- ↑ Taning, Clauvis Nji Tizi; Van Eynde, Benigna; Yu, Na; Ma, Sanyuan; Smagghe, Guy (April 2017). "CRISPR/Cas9 in insects: Applications, best practices and biosafety concerns". Journal of Insect Physiology 98: 245–257. doi:10.1016/j.jinsphys.2017.01.007. PMID 28108316.
- ↑ Dong, Yuemei; Simões, Maria L.; Marois, Eric; Dimopoulos, George (2018-03-08). "CRISPR/Cas9 -mediated gene knockout of Anopheles gambiae FREP1 suppresses malaria parasite infection". PLOS Pathogens 14 (3): e1006898. doi:10.1371/journal.ppat.1006898. PMID 29518156.
- ↑ Gallagher, James (31 May 2019). "GM fungus rapidly kills 99% of malaria mosquitoes, study suggests". BBC News Online. https://www.bbc.com/news/health-48464510.
External links
- Anopheles Database
- Anopheles gambiae Genome and Related Data
- CDC – National Center for Infectious Diseases, Division of Parasitic Diseases; Malaria
- CDC – World map showing distribution of various Anopheles species
- Walter Reed Biosystematics Unit. – Links to the online mosquito catalog, keys for mosquito identification, images and information on medically important species and much more.
- Malaria Atlas Project
- Anopheles gambiae taxonomy, facts and life cycle
- Anopheles quadrimaculatus, common malaria mosquito on the University of Florida / Institute of Food and Agricultural Sciences Featured Creatures website
- http://animaldiversity.ummz.umich.edu/site/accounts/classification/Anopheles.html
Wikidata ☰ Q158597 entry
 |