Biology:B-cell linker
 Generic protein structure example |
B-cell linker (BLNK) protein is expressed in B cells and macrophages and plays a large role in B cell receptor signaling.[1] Like all adaptor proteins, BLNK has no known intrinsic enzymatic activity.[2] Its function is to temporally and spatially coordinate and regulate downstream signaling effectors in B cell receptor (BCR) signaling, which is important in B cell development.[3] Binding of these downstream effectors is dependent on BLNK phosphorylation.[4][5] BLNK is encoded by the BLNK gene[4][6] and is also known as SLP-65,[7] BASH,[8] and BCA.[9]
Structure and localization
BLNK consists of a N-terminal leucine zipper motif followed by an acidic region, a proline-rich region, and a C-terminal SH2 domain.[10][1] The leucine zipper motif allows BLNK to localize to the plasma membrane, presumably by coiled-coil interactions with a membrane protein.[1] This leucine zipper motif distinguishes BLNK from lymphoctye cytosolic protein 2, also known as LCP-2 or SLP-76, which plays a similar role in T cell receptor signaling.[11] Although LCP-2 has an N-terminal heptad-like organization of leucine and isoleucine residues like BLNK, it has not been experimentally shown to have the leucine zipper motif.[12] Recruitment of BLNK to the plasma membrane is also achieved by binding of the SH2 domain of BLNK to a non-ITAM phospho-tyrosine on the cytoplasmic domain of CD79A, which is a part of Igα and the B cell receptor complex.[13][14][15]
Function
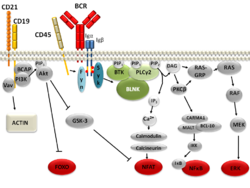
BLNK's function and importance in B cell development were first illustrated in BLNK deficient DT40 cells, a chicken B cell line.[3] DT40 cells had interrupted B cell development: there was no calcium mobilization response in the B cell, impaired activation of the mitogen-activated protein (MAP) kinases p38, JNK, and somewhat inhibited ERK activation upon (BCR) activation as compared to wild type DT40 cells.[3] In knockout mice, BLNK deficiency results in a partial block in B cell development,[16][17] and in humans BLNK deficiency results in a much more profound block in B cell development.[18][1]
Linker or adaptor proteins provide mechanisms by which receptors can amplify and regulate downstream effector proteins.[2] BLNK is essential for normal B-cell development as part of the B cell receptor signaling pathway. [supplied by OMIM][6][19][20]
Evidence also suggests that BLNK may have tumor suppressive activity through its interaction with Bruton's tyrosine kinase (Btk) [21][22] and regulation of the pre-B cell checkpoint.[10][23]
Phosphorylation and interactions
The acidic region of BLNK contains several inducibly phosphorylated tyrosine residues, at least five of which are found in humans.[24] Evidence suggests that BLNK is phosphorylated by the tyrosine-protein kinase Syk after B cell receptor activation.[4][5][20][25] Phosphorylation of these residues provides docking sites necessary for downstream protein-protein interactions between BLNK and the SH2 domain-containing proteins Grb2,[4][7][13][26] PLCG2, Btk, the Vav protein family, and Nck.[27][5][4] BLNK has also been shown to interact with SH3KBP1[28] and MAP4K1.[29] A more recent mass spectrometry study of BLNK in DT40 cells found that at least 41 unique serine, threonine, and tyrosine residues are phosphorylated on BLNK.[30]
References
- ↑ 1.0 1.1 1.2 1.3 "A leucine zipper in the N terminus confers membrane association to SLP-65". Nature Immunology 6 (2): 204–210. February 2005. doi:10.1038/ni1163. PMID 15654340.
- ↑ 2.0 2.1 "Adaptor proteins: Flexible and dynamic modulators of immune cell signalling". Scandinavian Journal of Immunology 92 (5): e12951. November 2020. doi:10.1111/sji.12951. PMID 32734639.
- ↑ 3.0 3.1 3.2 "BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells". Immunity 10 (1): 117–125. January 1999. doi:10.1016/S1074-7613(00)80012-6. PMID 10023776.
- ↑ 4.0 4.1 4.2 4.3 4.4 "BLNK: a central linker protein in B cell activation". Immunity 9 (1): 93–103. July 1998. doi:10.1016/S1074-7613(00)80591-9. PMID 9697839.
- ↑ 5.0 5.1 5.2 "Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase" (in English). The Journal of Biological Chemistry 277 (35): 31703–31714. August 2002. doi:10.1074/jbc.M201362200. PMID 12077122.
- ↑ 6.0 6.1 "Entrez Gene: BLNK B-cell linker". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=29760.
- ↑ 7.0 7.1 "SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation". The Journal of Experimental Medicine 188 (4): 791–795. August 1998. doi:10.1084/jem.188.4.791. PMID 9705962.
- ↑ "BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius". Journal of Immunology 161 (11): 5804–5808. December 1998. doi:10.4049/jimmunol.161.11.5804. PMID 9834055.
- ↑ "bca: an activation-related B-cell gene". Molecular Immunology 35 (1): 55–63. January 1998. doi:10.1016/s0161-5890(98)00008-x. PMID 9683264.
- ↑ 10.0 10.1 "Dual role of the adaptor protein SLP-65: organizer of signal transduction and tumor suppressor of pre-B cell leukemia". Immunologic Research 34 (2): 143–155. 2006. doi:10.1385/ir:34:2:143. PMID 16760574.
- ↑ "SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond". Nature Reviews. Immunology 6 (1): 67–78. January 2006. doi:10.1038/nri1750. PMID 16493428.
- ↑ "Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation". Immunological Reviews 192 (1): 32–41. April 2003. doi:10.1034/j.1600-065X.2003.00005.x. PMID 12670393.
- ↑ 13.0 13.1 "Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-alpha". European Journal of Immunology 31 (7): 2126–2134. July 2001. doi:10.1002/1521-4141(200107)31:7<2126::AID-IMMU2126>3.0.CO;2-O. PMID 11449366.
- ↑ "The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways". Molecular and Cellular Biology 22 (8): 2524–2535. April 2002. doi:10.1128/MCB.22.8.2524-2535.2002. PMID 11909947.
- ↑ "Dual requirement for the Ig alpha immunoreceptor tyrosine-based activation motif (ITAM) and a conserved non-Ig alpha ITAM tyrosine in supporting Ig alpha beta-mediated B cell development". Journal of Immunology 174 (4): 2012–2020. February 2005. doi:10.4049/jimmunol.174.4.2012. PMID 15699130.
- ↑ "Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65". Immunity 11 (5): 547–554. November 1999. doi:10.1016/S1074-7613(00)80130-2. PMID 10591180.
- ↑ "Requirement for B cell linker protein (BLNK) in B cell development". Science 286 (5446): 1949–1954. December 1999. doi:10.1126/science.286.5446.1949. PMID 10583957.
- ↑ "An essential role for BLNK in human B cell development". Science 286 (5446): 1954–1957. December 1999. doi:10.1126/science.286.5446.1954. PMID 10583958.
- ↑ "B-cell antigen-receptor signalling in lymphocyte development". Immunology 110 (4): 411–420. December 2003. doi:10.1111/j.1365-2567.2003.01756.x. PMID 14632637.
- ↑ 20.0 20.1 Janeway's immunobiology. Paul Travers, Mark Walport, Charles Janeway (8th ed.). New York: Garland Science. 2012. ISBN 978-0-8153-4243-4. OCLC 733935898. https://www.worldcat.org/oclc/733935898.
- ↑ "Cbl-b positively regulates Btk-mediated activation of phospholipase C-gamma2 in B cells". The Journal of Experimental Medicine 196 (1): 51–63. July 2002. doi:10.1084/jem.20020068. PMID 12093870.
- ↑ "Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling". Blood 94 (7): 2357–2364. October 1999. doi:10.1182/blood.V94.7.2357.419k40_2357_2364. PMID 10498607.
- ↑ "Involvement of SLP-65 and Btk in tumor suppression and malignant transformation of pre-B cells". Seminars in Immunology 18 (1): 67–76. February 2006. doi:10.1016/j.smim.2005.10.002. PMID 16300960.
- ↑ "BLNK B cell linker [Homo sapiens (human) - Gene - NCBI"] (in en). https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=29760.
- ↑ "Syk and pTyr'd: Signaling through the B cell antigen receptor". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1793 (7): 1115–1127. July 2009. doi:10.1016/j.bbamcr.2009.03.004. PMID 19306898.
- ↑ "BLNK is associated with the CD72/SHP-1/Grb2 complex in the WEHI231 cell line after membrane IgM cross-linking". European Journal of Immunology 30 (5): 1326–1330. May 2000. doi:10.1002/(SICI)1521-4141(200005)30:5<1326::AID-IMMU1326>3.0.CO;2-Q. PMID 10820378.
- ↑ "BLNK: molecular scaffolding through 'cis'-mediated organization of signaling proteins". The EMBO Journal 21 (23): 6461–6472. December 2002. doi:10.1093/emboj/cdf658. PMID 12456653.
- ↑ "Characterization of the CIN85 adaptor protein and identification of components involved in CIN85 complexes". Biochemical and Biophysical Research Communications 278 (1): 167–174. November 2000. doi:10.1006/bbrc.2000.3760. PMID 11071869. https://zenodo.org/record/1229524.
- ↑ "B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1". The Journal of Experimental Medicine 194 (4): 529–539. August 2001. doi:10.1084/jem.194.4.529. PMID 11514608.
- ↑ "SLP-65 phosphorylation dynamics reveals a functional basis for signal integration by receptor-proximal adaptor proteins". Molecular & Cellular Proteomics 8 (7): 1738–1750. July 2009. doi:10.1074/mcp.M800567-MCP200. PMID 19372136.
Further reading
- "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene 138 (1–2): 171–174. January 1994. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- "Identification of two tyrosine phosphoproteins, pp70 and pp68, which interact with phospholipase Cgamma, Grb2, and Vav after B cell antigen receptor activation". The Journal of Biological Chemistry 272 (43): 27362–27368. October 1997. doi:10.1074/jbc.272.43.27362. PMID 9341187.
- "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene 200 (1–2): 149–156. October 1997. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- "SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation". The Journal of Experimental Medicine 188 (4): 791–795. August 1998. doi:10.1084/jem.188.4.791. PMID 9705962.
- "Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling". Blood 94 (7): 2357–2364. October 1999. doi:10.1182/blood.V94.7.2357.419k40_2357_2364. PMID 10498607.
- "Interaction of SLP adaptors with the SH2 domain of Tec family kinases". European Journal of Immunology 29 (11): 3702–3711. November 1999. doi:10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. PMID 10556826.
- "BLNK is associated with the CD72/SHP-1/Grb2 complex in the WEHI231 cell line after membrane IgM cross-linking". European Journal of Immunology 30 (5): 1326–1330. May 2000. doi:10.1002/(SICI)1521-4141(200005)30:5<1326::AID-IMMU1326>3.0.CO;2-Q. PMID 10820378.
- "Src homology region 2 (SH2) domain-containing phosphatase-1 dephosphorylates B cell linker protein/SH2 domain leukocyte protein of 65 kDa and selectively regulates c-Jun NH2-terminal kinase activation in B cells". Journal of Immunology 165 (3): 1344–1351. August 2000. doi:10.4049/jimmunol.165.3.1344. PMID 10903736.
- "Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex". Immunity 13 (2): 243–253. August 2000. doi:10.1016/S1074-7613(00)00024-8. PMID 10981967.
- "Characterization of the CIN85 adaptor protein and identification of components involved in CIN85 complexes". Biochemical and Biophysical Research Communications 278 (1): 167–174. November 2000. doi:10.1006/bbrc.2000.3760. PMID 11071869. https://zenodo.org/record/1229524.
- "The adaptor protein BLNK is required for b cell antigen receptor-induced activation of nuclear factor-kappa B and cell cycle entry and survival of B lymphocytes". The Journal of Biological Chemistry 276 (23): 20055–20063. June 2001. doi:10.1074/jbc.M010800200. PMID 11274146.
- "SHP-1 requires inhibitory co-receptors to down-modulate B cell antigen receptor-mediated phosphorylation of cellular substrates". The Journal of Biological Chemistry 276 (28): 26648–26655. July 2001. doi:10.1074/jbc.M100997200. PMID 11356834.
- "Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-alpha". European Journal of Immunology 31 (7): 2126–2134. July 2001. doi:10.1002/1521-4141(200107)31:7<2126::AID-IMMU2126>3.0.CO;2-O. PMID 11449366.
- "Hematopoietic progenitor kinase 1 associates physically and functionally with the adaptor proteins B cell linker protein and SLP-76 in lymphocytes". The Journal of Biological Chemistry 276 (48): 45207–45216. November 2001. doi:10.1074/jbc.M106811200. PMID 11487585.
- "Epstein-Barr virus latent membrane protein 2A (LMP2A) employs the SLP-65 signaling module". The Journal of Experimental Medicine 194 (3): 255–264. August 2001. doi:10.1084/jem.194.3.255. PMID 11489945.
- "B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1". The Journal of Experimental Medicine 194 (4): 529–539. August 2001. doi:10.1084/jem.194.4.529. PMID 11514608.
- "The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways". Molecular and Cellular Biology 22 (8): 2524–2535. April 2002. doi:10.1128/MCB.22.8.2524-2535.2002. PMID 11909947.
- "Cbl-b positively regulates Btk-mediated activation of phospholipase C-gamma2 in B cells". The Journal of Experimental Medicine 196 (1): 51–63. July 2002. doi:10.1084/jem.20020068. PMID 12093870.
External links
- Human BLNK genome location and BLNK gene details page in the UCSC Genome Browser.
 |
