Biology:SH2 domain
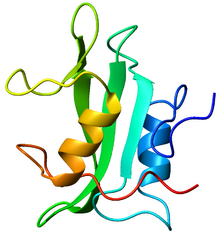 Crystallographic structure of the SH2 domain. The structure consists of a large beta sheet (green) flanked by two alpha-helices (orange and blue).[1] | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | SH2 | ||||||||
| Pfam | PF00017 | ||||||||
| InterPro | IPR000980 | ||||||||
| SMART | SH2 | ||||||||
| PROSITE | PDOC50001 | ||||||||
| SCOP2 | 1sha / SCOPe / SUPFAM | ||||||||
| CDD | cd00173 | ||||||||
| |||||||||
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein[2] and in many other intracellular signal-transducing proteins.[3] SH2 domains bind to phosphorylated tyrosine residues on other proteins, modifying the function or activity of the SH2-containing protein. The SH2 domain may be considered the prototypical modular protein-protein interaction domain, allowing the transmission of signals controlling a variety of cellular functions.[4] SH2 domains are especially common in adaptor proteins that aid in the signal transduction of receptor tyrosine kinase pathways.[5]
Structure and interactions
SH2 domains contain about 100 amino acid residues and exhibit a central antiparallel β-sheet centered between two α-helices.[6] Binding to phosphotyrosine-containing peptides involves a strictly-conserved Arg residue that pairs with the negatively-charged phosphate on the phosphotyrosine,[7] and a surrounding pocket that recognizes flanking sequences on the target peptide.[6][7] Compared to other signaling proteins, SH2 domains exhibit only a moderate degree of specificity for their target peptides, due to the relative weakness of the interactions with the flanking sequences.[8]
Over 100 human proteins are known to contain SH2 domains.[9] A variety of tyrosine-containing sequences have been found to bind SH2 domains and are conserved across a wide range of organisms, performing similar functions.[10] Binding of a phosphotyrosine-containing protein to an SH2 domain may lead to either activation or inactivation of the SH2-containing protein, depending on the types of interactions formed between the SH2 domain and other domains of the enzyme. Mutations that disrupt the structural stability of the SH2 domain, or that affect the binding of the phosphotyrosine peptide of the target, are involved in a range of diseases including X-linked agammaglobulinemia and severe combined immunodeficiency.[11]
Diversity
SH2 domains are not present in yeast and appear at the boundary between protozoa and animalia in organisms such as the social amoeba Dictyostelium discoideum.[12]
A detailed bioinformatic examination of SH2 domains of human and mouse reveals 120 SH2 domains contained within 115 proteins encoded by the human genome,[13] representing a rapid rate of evolutionary expansion among the SH2 domains.
A large number of SH2 domain structures have been solved and many SH2 proteins have been knocked out in mice.
Applications
SH2 domains, and other binding domains, have been used in protein engineering to create protein assemblies. Protein assemblies are formed when several proteins bind to one another to create a larger structure (called a supramolecular assembly). Using molecular biology techniques, fusion proteins of specific enzymes and SH2 domains have been created, which can bind to each other to form protein assemblies.
Since SH2 domains require phosphorylation in order for binding to occur, the use of kinase and phosphatase enzymes gives researchers control over whether protein assemblies will form or not. High affinity engineered SH2 domains have been developed and utilized for protein assembly applications.[14]
The goal of most protein assembly formation is to increase the efficiency of metabolic pathways via enzymatic co-localization.[15] Other applications of SH2 domain mediated protein assemblies have been in the formation of high density fractal-like structures, which have extensive molecular trapping properties.[16]
Examples
Human proteins containing this domain include:
- ABL1; ABL2
- BCAR3; BLK; BLNK; BMX; BTK
- CHN2; CISH; CRK; CRKL; CSK
- DAPP1
- FER; FES; FGR; FRK; FYN
- GRAP; GRAP2; GRB10; GRB14; GRB2; GRB7
- HCK; HSH2D
- INPP5D; INPPL1; ITK; JAK2; LCK; LCP2; LYN
- MATK; NCK1; NCK2
- PIK3R1; PIK3R2; PIK3R3; PLCG1; PLCG2; PTK6; PTPN11; PTPN6; RASA1
- SH2B1; SH2B2; SH2B3; SH2D1A; SH2D1B; SH2D2A; SH2D3A; SH2D3C; SH2D4A; SH2D4B; SH2D5; SH2D6; SH3BP2; SHB; SHC1; SHC3; SHC4; SHD; SHE
- SLA; SLA2
- SOCS1; SOCS2; SOCS3; SOCS4; SOCS5; SOCS6; SOCS7
- SRC; SRMS
- STAT1; STAT2; STAT3; STAT4; STAT5A; STAT5B; STAT6
- SUPT6H; SYK
- TEC; TENC1; TNS; TNS1; TNS3; TNS4; TXK
- VAV1; VAV2; VAV3
- YES1; ZAP70
See also
- Phosphotyrosine-binding domains also bind phosphorylated tyrosines
- Anthony Pawson, discoverer of the SH2 Domain
References
- ↑ PDB: 1lkk; "Crystal structures of the human p56lck SH2 domain in complex with two short phosphotyrosyl peptides at 1.0 A and 1.8 A resolution". Journal of Molecular Biology 256 (3): 601–10. March 1996. doi:10.1006/jmbi.1996.0112. PMID 8604142.
- ↑ "A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps". Molecular and Cellular Biology 6 (12): 4396–408. December 1986. doi:10.1128/mcb.6.12.4396. PMID 3025655.
- ↑ "Conservation analysis and structure prediction of the SH2 family of phosphotyrosine binding domains". FEBS Letters 304 (1): 15–20. June 1992. doi:10.1016/0014-5793(92)80579-6. PMID 1377638.
- ↑ "SH2 domains, interaction modules and cellular wiring". Trends in Cell Biology 11 (12): 504–511. December 2001. doi:10.1016/s0962-8924(01)02154-7. PMID 11719057.
- ↑ "Phosphotyrosine signaling proteins that drive oncogenesis tend to be highly interconnected". Molecular & Cellular Proteomics 12 (5): 1204–13. May 2013. doi:10.1074/mcp.M112.025858. PMID 23358503.
- ↑ 6.0 6.1 "Src-homology 2 domains: Structure, mechanisms, and drug discovery". Biopolymers (Peptide Science) 47 (3): 243–261. 1998. doi:10.1002/(SICI)1097-0282(1998)47:3<243::AID-BIP4>3.0.CO;2-P. PMID 9817027.
- ↑ 7.0 7.1 "Sequence, structure, and energetic determinants of phosphopeptide selectivity of SH2 domains". Journal of Molecular Biology 334 (4): 823–841. 2003. doi:10.1016/j.jmb.2003.09.075. PMID 14636606.
- ↑ "Molecular recognition by SH2 domains". Advances in Protein Chemistry 61: 161–210. 2002. doi:10.1016/s0065-3233(02)61005-8. ISBN 9780120342618. PMID 12461824.
- ↑ "The SH2 domain-containing proteins in 21 species establish the provenance and scope of phosphotyrosine signaling in eukaryotes". Science Signaling 4 (202): ra83. December 2011. doi:10.1126/scisignal.2002105. PMID 22155787.
- ↑ "The conservation pattern of short linear motifs is highly correlated with the function of interacting protein domains". BMC Genomics 9: 452. October 2008. doi:10.1186/1471-2164-9-452. PMID 18828911.
- ↑ "SH2 domains: Modulators of nonreceptor tyrosine kinase activity". Current Opinion in Structural Biology 19 (6): 643–649. December 2009. doi:10.1016/j.sbi.2009.10.001. PMID 19926274.
- ↑ "The genome of the social amoeba Dictyostelium discoideum". Nature 435 (7038): 43–57. May 2005. doi:10.1038/nature03481. PMID 15875012. Bibcode: 2005Natur.435...43E.
- ↑ "The human and mouse complement of SH2 domain proteins-establishing the boundaries of phosphotyrosine signaling". Molecular Cell 22 (6): 851–68. June 2006. doi:10.1016/j.molcel.2006.06.001. PMID 16793553.
- ↑ Kaneko, T.; Huang, H.; Cao, X.; Li, X.; Li, C.; Voss, C.; Sidhu, S. S.; Li, S. S. C. (2012-09-25). "Superbinder SH2 Domains Act as Antagonists of Cell Signaling" (in en). Science Signaling 5 (243): ra68. doi:10.1126/scisignal.2003021. ISSN 1945-0877. PMID 23012655.
- ↑ Yang, Lu; Dolan, E.M.; Tan, S.K.; Lin, T.; Sontag, E.D.; Khare, S.D. (2017). "Computation-Guided Design of a Stimulus-Responsive Multienzyme Supramolecular Assembly". ChemBioChem 18 (20): 2000–2006. doi:10.1002/cbic.201700425. ISSN 1439-7633. PMID 28799209.
- ↑ Hernández N.E., Hansen W.A., Zhu D., Shea M.E., Khalid M., Manichev V., Putnins M., Chen M., Dodge A.G., Yang L., Marrero-Berríos I., Banal M., Rechani P., Gustafsson T., Feldman L.C., Lee S-.H., Wackett L.P., Dai W., Khare S.D. (2019). Stimulus-responsive self-assembly of protein-based fractals by computational design. Nat. Chem. 2019 11(7): 605-614. Pre-print available at bioRxiv doi: 10.1101/274183.
External links
- SH2 Domain website created by lab of Dr. Piers Nash
 |
