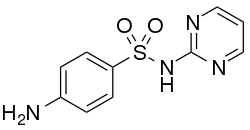
Chemistry:Sulfadiazine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682130 |
| Pregnancy category |
|
| Routes of administration | Topical cream, by mouth |
| Drug class | Antibiotic (sulfonamide)[2] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 38-48%[2] |
| Metabolism | Liver (acetylation)[2] |
| Elimination half-life | 7-17 hours [2] |
| Excretion | Urine [2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H10N4O2S |
| Molar mass | 250.28 g·mol−1 |
| Melting point | 252 to 256 °C (486 to 493 °F) |
| | |
Sulfadiazine is an antibiotic.[2] Used together with pyrimethamine, a dihydrofolate reductase inhibitor, it is the treatment of choice for toxoplasmosis, which is caused by a protozoan parasite.[3] It is a second-line treatment for otitis media, prophylaxis of rheumatic fever, chancroid, chlamydia, and infections by Haemophilus influenzae.[2] It is also used as adjunct therapy for chloroquine-resistant malaria and several forms of bacterial meningitis.[4] It is taken by mouth.[2] Sulfadiazine is available in multiple generic tablets of 500 mg. For urinary tract infections, the usual dose is 4 to 6 grams daily in 3 to 6 divided doses.[4]
Common side effects include nausea, diarrhea, headache, fever, rash, depression, and pancreatitis.[2] It should not be used in people who have severe liver problems, kidney problems, or porphyria.[3] If used during pregnancy, it may increase the risk of kernicterus in the baby.[2] While the company that makes it does not recommend use during breastfeeding, use is believed to be safe if the baby is otherwise healthy.[1] It is in the sulfonamide class of medications.[2]
Sulfadiazine was approved for medical use in the United States in 1941.[2][5] It is on the World Health Organization's List of Essential Medicines.[6] Sulfadiazine is available as a generic medication.[2]
Medical uses
It eliminates bacteria that cause infections by stopping the production of folate inside the bacterial cell, and is commonly used to treat urinary tract infections and burns.
In combination, sulfadiazine and pyrimethamine can be used to treat toxoplasmosis, the disease caused by Toxoplasma gondii.
Other uses
Sulfadiazine is used in plant research for selecting and maintaining genetically manipulated cells.[7]
Mechanism of action
Sulfadiazine works by inhibiting the enzyme dihydropteroate synthetase.
Side effects
Side effects reported for sulfadiazine include nausea, loss of appetite, dizziness, gastrointestinal upset, rash and fever.[4]
Brand names
This drug is sold branded as Lantrisul, Neotrizine, Sulfa-Triple #2, Sulfadiazine, Sulfaloid, Sulfonamides Duplex, Sulfose, Terfonyl, Triple Sulfa, Triple Sulfas, and Triple Sulfoid.
See also
References
- ↑ 1.0 1.1 "Sulfadiazine Use During Pregnancy | Drugs.com". https://www.drugs.com/pregnancy/sulfadiazine.html.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "Sulfadiazine". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/sulfadiazine.html.
- ↑ 3.0 3.1 WHO Model Formulary 2008. World Health Organization. 2009. pp. 126, 205. ISBN 9789241547659.
- ↑ 4.0 4.1 4.2 "Sulfadiazine", LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases), 2012, PMID 31643992, http://www.ncbi.nlm.nih.gov/books/NBK548681/, retrieved 2021-12-27
- ↑ "Drugs@FDA: FDA Approved Drug Products". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=reportsSearch.process&rptName=2&reportSelectMonth=8&reportSelectYear=1941&nav.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Sulfadiazine and phosphinothricin selection systems optimised for the transformation of tobacco BY-2 cells". Plant Cell Reports (Springer Science and Business Media LLC) 42 (3): 535–548. March 2023. doi:10.1007/s00299-022-02975-7. PMID 36609768.
External links
- "Sulfadiazine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/sulfadiazine.
 |

