Biology:Dynactin
Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).[1][2]
Discovery
Dynactin was identified as an activity that allowed purified cytoplasmic dynein to move membrane vesicles along microtubules in vitro.[3] It was shown to be a multiprotein complex and named "dynactin" because of its role in dynein activation.[4]
The main features of dynactin were visualized by quick-freeze, deep-etch, rotary shadow electron microscopy. It appears as a short filament, 37-nm in length, which resembles F-actin, plus a thinner, laterally oriented arm.[5] Antibody labelling was used to map the location of the dynactin subunits.[5][6]
Structure
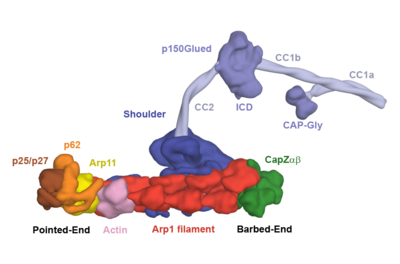
Dynactin consists of three major structural domains: (1) sidearm-shoulder: DCTN1/p150Glued, DCTN2/p50/dynamitin, DCTN3/p24/p22;(2)the Arp1 filament: ACTR1A/Arp1/centractin, actin, CapZ; and (3) the pointed end complex: Actr10/Arp11, DCTN4/p62, DCTN5/p25, and DCTN6/p27.[1]
A 4Å cryo-EM structure of dynactin [7] revealed that its filament contains eight Arp1 molecules, one β-actin and one Arp11. In the pointed end complex p62/DCTN4 binds to Arp11 and β-actin and p25 and p27 bind both p62 and Arp11. At the barbed end the capping protein (CapZαβ) binds the Arp1 filament in the same way that it binds actin, although with more charge complementarity, explaining why it binds dynactin more tightly than actin.[8]
The shoulder contains two copies of p150Glued/DCTN1, four copies of p50/DCTN2 and two copies of p24/DCTN3.[1] These proteins form long bundles of alpha helices, which wrap over each other and contact the Arp1 filament.[7] The N-termini of p50/DCTN2 emerge from the shoulder and coat the filament, providing a mechanism for controlling the filament length.[7] The C-termini of the p150Glued/DCTN1 dimer are embedded in the shoulder, whereas the N-terminal 1227 amino acids form the projecting arm. The arm consists of an N-terminal CAPGly domain which can bind the C-terminal tails of microtubules and the microtubule plus end binding protein EB1. This is followed by a basic region, also involved in microtubule binding, a folded-back coiled coil (CC1), the intercoiled domain (ICD) and a second coiled coil domain (CC2).[7] The p150Glued arm can dock into against the side of the Arp1 filament and pointed end complex.[7]
DCTN2 (dynamitin) is also involved in anchoring microtubules to centrosomes and may play a role in synapse formation during brain development.[9] Arp1 has been suggested as the domain for dynactin binding to membrane vesicles (such as Golgi or late endosome) through its association with β-spectrin.[10][11][12][13] The pointed end complex (PEC) has been shown to be involved in selective cargo binding. PEC subunits p62/DCTN4 and Arp11/Actr10 are essential for dynactin complex integrity and dynactin/dynein targeting to the nuclear envelope before mitosis.[14][15][16] Actr10 along with Drp1 (Dynamin related protein 1) have been documented as vital to the attachment of mitochondria to the dynactin complex.[17] Dynactin p25/DCTN5 and p27/DCTN6 are not essential for dynactin complex integrity, but are required for early and recycling endosome transport during the interphase and regulation of the spindle assembly checkpoint in mitosis.[16][18][19]
Interaction with dynein
Dynein and dynactin were reported to interact directly by the binding of dynein intermediate chains with p150Glued.[20] The affinity of this interaction is around 3.5μM.[21] Dynein and dynactin do not run together in a sucrose gradient, but can be induced to form a tight complex in the presence of the N-terminal 400 amino acids of Bicaudal D2 (BICD2), a cargo adaptor that links dynein and dynactin to Golgi derived vesicles.[22] In the presence of BICD2, dynactin binds to dynein and activates it to move for long distances along microtubules.[23][24]
A cryo-EM structure of dynein, dynactin and BICD2 [7] showed that the BICD2 coiled coil runs along the dynactin filament. The tail of dynein also binds to the Arp1 filament, sitting in the equivalent site that myosin uses to bind actin. The contacts between the dynein tail and dynactin all involve BICD, explaining why it is needed to bring them together. The dynein/dynactin/BICD2 (DDB) complex has also been observed, by negative stain EM, on microtubules. This shows that the cargo (Rab6) binding end of BICD2 extends out through the pointed end complex at the opposite end away from the dynein motor domains.[25]
Functions
Dynactin is often essential for dynein activity[1][3] and can be thought of as a "dynein receptor"[20] that modulates binding of dynein to cell organelles which are to be transported along microtubules.[26][27] Dynactin also enhances the processivity of cytoplasmic dynein[28] and kinesin-2 motors.[29] Dynactin is involved in various processes like chromosome alignment and spindle organization[30] in cell division.[31] Dynactin contributes to mitotic spindle pole focusing through its binding to nuclear mitotic apparatus protein (NuMA).[32][33] Dynactin also targets to the kinetochore through binding between DCTN2/dynamitin and zw10 and has a role in mitotic spindle checkpoint inactivation.[34][35] During prometaphase, dynactin also helps target polo-like kinase 1 (Plk1) to kinetochores through cyclin dependent kinase 1 (Cdk1)-phosphorylated DCTN6/p27, which is involved in proper microtubule-kinetochore attachment and recruitment of spindle assembly checkpoint protein Mad1.[19] In addition, dynactin has been shown to play an essential role in maintaining nuclear position in Drosophila,[36] zebrafish[37] or in different fungi.[38][39] Dynein and dynactin concentrate on the nuclear envelope during the prophase and facilitate nuclear envelope breakdown via its DCTN4/p62 and Arp11 subunits.[16][14] Dynactin is also required for microtubule anchoring at centrosomes and centrosome integrity.[40] Destabilization of the centrosomal pool of dynactin also causes abnormal G1 centriole separation and delayed entry into S phase, suggesting that dynactin contributes to the recruitment of important cell cycle regulators to centrosomes.[41] In addition to transport of various organelles in the cytoplasm, dynactin also links kinesin II to organelles.[42]
See also
- Motor protein
- Dynein
- DCTN1
- Centractin
References
- ↑ 1.0 1.1 1.2 1.3 "Dynactin". Annual Review of Cell and Developmental Biology 20: 759–79. November 2004. doi:10.1146/annurev.cellbio.20.012103.094623. PMID 15473859.
- ↑ "How dynein and dynactin transport cargos: a structural perspective". Current Opinion in Structural Biology 37: 62–70. April 2016. doi:10.1016/j.sbi.2015.12.003. PMID 26773477.
- ↑ 3.0 3.1 "Two activators of microtubule-based vesicle transport". The Journal of Cell Biology 115 (5): 1309–18. December 1991. doi:10.1083/jcb.115.5.1309. PMID 1835460.
- ↑ "Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein". The Journal of Cell Biology 115 (6): 1639–50. December 1991. doi:10.1083/jcb.115.6.1639. PMID 1836789.
- ↑ 5.0 5.1 "Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin". The Journal of Cell Biology 126 (2): 403–12. July 1994. doi:10.1083/jcb.126.2.403. PMID 7518465.
- ↑ "Analysis of dynactin subcomplexes reveals a novel actin-related protein associated with the arp1 minifilament pointed end". The Journal of Cell Biology 147 (2): 307–20. October 1999. doi:10.1083/jcb.147.2.307. PMID 10525537.
- ↑ "Dynactin integrity depends upon direct binding of dynamitin to Arp1". Molecular Biology of the Cell 25 (14): 2171–80. July 2014. doi:10.1091/mbc.E14-03-0842. PMID 24829381.
- ↑ "Interaction of Cep135 with a p50 dynactin subunit in mammalian centrosomes". Cell Motility and the Cytoskeleton 58 (1): 53–66. May 2004. doi:10.1002/cm.10175. PMID 14983524.
- ↑ "Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles". The Journal of Cell Biology 135 (6 Pt 2): 1815–29. December 1996. doi:10.1083/jcb.135.6.1815. PMID 8991093.
- ↑ "beta III spectrin binds to the Arp1 subunit of dynactin". The Journal of Biological Chemistry 276 (39): 36598–605. September 2001. doi:10.1074/jbc.M104838200. PMID 11461920.
- ↑ "Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids". Molecular Cell 7 (1): 173–83. January 2001. doi:10.1016/S1097-2765(01)00165-4. PMID 11172722.
- ↑ "Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin". The Journal of Cell Biology 176 (4): 459–71. February 2007. doi:10.1083/jcb.200606077. PMID 17283181.
- ↑ 14.0 14.1 "Cytoplasmic dynein as a facilitator of nuclear envelope breakdown". Cell 108 (1): 97–107. January 2002. doi:10.1016/S0092-8674(01)00628-6. PMID 11792324.
- ↑ "Arp11 affects dynein-dynactin interaction and is essential for dynein function in Aspergillus nidulans". Traffic 9 (7): 1073–87. July 2008. doi:10.1111/j.1600-0854.2008.00748.x. PMID 18410488.
- ↑ 16.0 16.1 16.2 "Dynactin's pointed-end complex is a cargo-targeting module". Molecular Biology of the Cell 23 (19): 3827–37. October 2012. doi:10.1091/mbc.E12-07-0496. PMID 22918948.
- ↑ Catherine Drerup, Amy Herbert, Kelly Monk, Alex Nechiporuk, "Regulation of mitochondria-dynactin interaction and mitochondrial retrograde transport in axons"
- ↑ "The p25 subunit of the dynactin complex is required for dynein-early endosome interaction". The Journal of Cell Biology 193 (7): 1245–55. June 2011. doi:10.1083/jcb.201011022. PMID 21708978.
- ↑ 19.0 19.1 "Dynactin helps target Polo-like kinase 1 to kinetochores via its left-handed beta-helical p27 subunit". The EMBO Journal 32 (7): 1023–35. April 2013. doi:10.1038/emboj.2013.30. PMID 23455152.
- ↑ 20.0 20.1 "Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued". The Journal of Cell Biology 131 (6 Pt 1): 1507–16. December 1995. doi:10.1083/jcb.131.6.1507. PMID 8522607.
- ↑ "Structural dynamics and multiregion interactions in dynein-dynactin recognition". The Journal of Biological Chemistry 286 (45): 39349–59. November 2011. doi:10.1074/jbc.M111.296277. PMID 21931160.
- ↑ "BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures". Molecular Biology of the Cell 23 (21): 4226–41. November 2012. doi:10.1091/mbc.E12-03-0210. PMID 22956769.
- ↑ "In vitro reconstitution of a highly processive recombinant human dynein complex". The EMBO Journal 33 (17): 1855–68. September 2014. doi:10.15252/embj.201488792. PMID 24986880.
- ↑ "Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes". Science 345 (6194): 337–41. July 2014. doi:10.1126/science.1254198. PMID 25035494. Bibcode: 2014Sci...345..337M.
- ↑ "Structural organization of the dynein-dynactin complex bound to microtubules". Nature Structural & Molecular Biology 22 (4): 345–7. April 2015. doi:10.1038/nsmb.2996. PMID 25751425.
- ↑ "The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport". Proceedings of the National Academy of Sciences of the United States of America 94 (22): 12180–5. October 1997. doi:10.1073/pnas.94.22.12180. PMID 9342383. Bibcode: 1997PNAS...9412180W.
- ↑ "Regulation of cytoplasmic dynein function in vivo by the Drosophila Glued complex". The Journal of Cell Biology 131 (2): 411–25. October 1995. doi:10.1083/jcb.131.2.411. PMID 7593168.
- ↑ "Dynactin increases the processivity of the cytoplasmic dynein motor". Nature Cell Biology 2 (1): 20–4. January 2000. doi:10.1038/71338. PMID 10620802.
- ↑ "Dynactin enhances the processivity of kinesin-2". Traffic 8 (2): 124–9. February 2007. doi:10.1111/j.1600-0854.2006.00517.x. PMID 17181772.
- ↑ "Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis". The Journal of Cell Biology 132 (4): 617–33. February 1996. doi:10.1083/jcb.132.4.617. PMID 8647893.
- ↑ "Cytoplasmic dynein and dynactin in cell division and intracellular transport". Current Opinion in Cell Biology 11 (1): 45–53. February 1999. doi:10.1016/S0955-0674(99)80006-4. PMID 10047518.
- ↑ "Opposing motor activities are required for the organization of the mammalian mitotic spindle pole". The Journal of Cell Biology 135 (2): 399–414. October 1996. doi:10.1083/jcb.135.2.399. PMID 8896597.
- ↑ "Formation of spindle poles by dynein/dynactin-dependent transport of NuMA". The Journal of Cell Biology 149 (4): 851–62. May 2000. doi:10.1083/jcb.149.4.851. PMID 10811826.
- ↑ "Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation". The Journal of Cell Biology 155 (7): 1159–72. December 2001. doi:10.1083/jcb.200105093. PMID 11756470.
- ↑ "ZW10 helps recruit dynactin and dynein to the kinetochore". The Journal of Cell Biology 142 (3): 763–74. August 1998. doi:10.1083/jcb.142.3.763. PMID 9700164.
- ↑ "Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons". Development 131 (19): 4677–86. October 2004. doi:10.1242/dev.01366. PMID 15329347.
- ↑ "Mechanism of positioning the cell nucleus in vertebrate photoreceptors". Proceedings of the National Academy of Sciences of the United States of America 104 (37): 14819–24. September 2007. doi:10.1073/pnas.0700178104. PMID 17785424. Bibcode: 2007PNAS..10414819T.
- ↑ "Dynamics of cytoplasmic dynein in living cells and the effect of a mutation in the dynactin complex actin-related protein Arp1". Current Biology 10 (10): 603–6. May 2000. doi:10.1016/S0960-9822(00)00488-7. PMID 10837229.
- ↑ "Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa". Proceedings of the National Academy of Sciences of the United States of America 93 (10): 4775–80. May 1996. doi:10.1073/pnas.93.10.4775. PMID 8643479. Bibcode: 1996PNAS...93.4775B.
- ↑ "Dynactin is required for microtubule anchoring at centrosomes". The Journal of Cell Biology 147 (2): 321–34. October 1999. doi:10.1083/jcb.147.2.321. PMID 10525538.
- ↑ "Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes". The Journal of Cell Biology 159 (2): 245–54. October 2002. doi:10.1083/jcb.200203089. PMID 12391026.
- ↑ "Dynactin is required for bidirectional organelle transport". The Journal of Cell Biology 160 (3): 297–301. February 2003. doi:10.1083/jcb.200210066. PMID 12551954.
Further reading
- "Brain disorder suggests common mechanism may underlie many neurodegenerative diseases". 11 January 2009. http://www.physorg.com/news150904281.html.
 |
