Biology:Prophase

Prophase (from grc προ- (pro-) 'before', and φάσις (phásis) 'appearance') is the first stage of cell division in both mitosis and meiosis. Beginning after interphase, DNA has already been replicated when the cell enters prophase. The main occurrences in prophase are the condensation of the chromatin reticulum and the disappearance of the nucleolus.[3]
Staining and microscopy
Microscopy can be used to visualize condensed chromosomes as they move through meiosis and mitosis.[4]
Various DNA stains are used to treat cells such that condensing chromosomes can be visualized as the move through prophase.[4]
The giemsa G-banding technique is commonly used to identify mammalian chromosomes, but utilizing the technology on plant cells was originally difficult due to the high degree of chromosome compaction in plant cells.[5][4] G-banding was fully realized for plant chromosomes in 1990.[6] During both meiotic and mitotic prophase, giemsa staining can be applied to cells to elicit G-banding in chromosomes.[2] Silver staining, a more modern technology, in conjunction with giesma staining can be used to image the synaptonemal complex throughout the various stages of meiotic prophase.[7] To perform G-banding, chromosomes must be fixed, and thus it is not possible to perform on living cells.[8]
Fluorescent stains such as DAPI can be used in both live plant and animal cells. These stains do not band chromosomes, but instead allow for DNA probing of specific regions and genes. Use of fluorescent microscopy has vastly improved spatial resolution.[9]
Mitotic prophase
Prophase is the first stage of mitosis in animal cells, and the second stage of mitosis in plant cells.[10] At the start of prophase there are two identical copies of each chromosome in the cell due to replication in interphase. These copies are referred to as sister chromatids and are attached by DNA element called the centromere.[11] The main events of prophase are: the condensation of chromosomes, the movement of the centrosomes, the formation of the mitotic spindle, and the beginning of nucleoli break down.[3]
Condensation of chromosomes
DNA that was replicated in interphase is condensed from DNA strands with lengths reaching 0.7 μm down to 0.2-0.3 μm.[3] This process employs the condensin complex.[11] Condensed chromosomes consist of two sister chromatids joined at the centromere.[12]
Movement of centrosomes
During prophase in animal cells, centrosomes move far enough apart to be resolved using a light microscope.[3] Microtubule activity in each centrosome is increased due to recruitment of γ-tubulin. Replicated centrosomes from interphase move apart towards opposite poles of the cell, powered by centrosome associated motor proteins.[13] Interdigitated interpolar microtubules from each centrosome interact with each other, helping to move the centrosomes to opposite poles.[13][3]
Formation of the mitotic spindle
Microtubules involved in the interphase scaffolding break down as the replicated centrosomes separate.[3] The movement of centrosomes to opposite poles is accompanied in animal cells by the organization of individual radial microtubule arrays (asters) by each centriole.[13] Interpolar microtubules from both centrosomes interact, joining the sets of microtubules and forming the basic structure of the mitotic spindle.[13] Plant cells do not have centrosomes and the chromosomes can nucleate microtubule assembly into the mitotic apparatus.[13] In plant cells, microtubules gather at opposite poles and begin to form the spindle apparatus at locations called foci.[10] The mitotic spindle is of great importance in the process of mitosis and will eventually segregate the sister chromatids in metaphase.[3]
Beginning of nucleoli breakdown
The nucleoli begin to break down in prophase, resulting in the discontinuation of ribosome production.[3] This indicates a redirection of cellular energy from general cellular metabolism to cellular division.[3] The nuclear envelope stays intact during this process.[10]
Meiotic prophase
Meiosis involves two rounds of chromosome segregation and thus undergoes prophase twice, resulting in prophase I and prophase II.[12] Prophase I is the most complex phase in all of meiosis because homologous chromosomes must pair and exchange genetic information.[3]:98 Prophase II is very similar to mitotic prophase.[12]
Prophase I
Prophase I is divided into five phases: leptotene, zygotene, pachytene, diplotene, and diakinesis. In addition to the events that occur in mitotic prophase, several crucial events occur within these phases such as pairing of homologous chromosomes and the reciprocal exchange of genetic material between these homologous chromosomes. Prophase I occurs at different speeds dependent on species and sex. Many species arrest meiosis in diplotene of prophase I until ovulation.[3]:98 In humans, decades can pass as oocytes remain arrested in prophase I only to quickly complete meiosis I prior to ovulation.[12]
Leptotene
In the first stage of prophase I, leptotene (from the Greek for "delicate"), chromosomes begin to condense. Each chromosome is in a diploid state and consists of two sister chromatids; however, the chromatin of the sister chromatids is not yet condensed enough to be resolvable in microscopy.[3]:98 Homologous regions within homologous chromosome pairs begin to associate with each other.[2]
Zygotene
In the second phase of prophase I, zygotene (from the Greek for "conjugation"), all maternally and paternally derived chromosomes have found their homologous partner.[3]:98 The homologous pairs then undergo synapsis, a process by which the synaptonemal complex (a proteinaceous structure) aligns corresponding regions of genetic information on maternally and paternally derived non-sister chromatids of homologous chromosome pairs.[3]:98[12] The paired homologous chromosome bound by the synaptonemal complex are referred to as bivalents or tetrads.[10][3]:98 Sex (X and Y) chromosomes do not fully synapse because only a small region of the chromosomes are homologous.[3]:98
The nucleolus moves from a central to a peripheral position in the nucleus.[14]
Pachytene
The third phase of prophase I, pachytene (from the Greek for "thick"), begins at the completion of synapsis.[3]:98 Chromatin has condensed enough that chromosomes can now be resolved in microscopy.[10] Structures called recombination nodules form on the synaptonemal complex of bivalents. These recombination nodules facilitate genetic exchange between the non-sister chromatids of the synaptonemal complex in an event known as crossing-over or genetic recombination.[3]:98 Multiple recombination events can occur on each bivalent. In humans, an average of 2-3 events occur on each chromosome.[13]:681
Diplotene
In the fourth phase of prophase I, diplotene (from the Greek for "twofold"), crossing-over is completed.[3]:99[10] Homologous chromosomes retain a full set of genetic information; however, the homologous chromosomes are now of mixed maternal and paternal descent.[3]:99 Visible junctions called chiasmata hold the homologous chromosomes together at locations where recombination occurred as the synaptonemal complex dissolves.[12][3]:99 It is at this stage where meiotic arrest occurs in many species.[3]:99
Diakinesis
In the fifth and final phase of prophase I, diakinesis (from the Greek for "double movement"), full chromatin condensation has occurred and all four sister chromatids can be seen in bivalents with microscopy. The rest of the phase resemble the early stages of mitotic prometaphase, as the meiotic prophase ends with the spindle apparatus beginning to form, and the nuclear membrane beginning to break down.[10][3]:99
Prophase II
Prophase II of meiosis is very similar to prophase of mitosis. The most noticeable difference is that prophase II occurs with a haploid number of chromosomes as opposed to the diploid number in mitotic prophase.[12][10] In both animal and plant cells chromosomes may de-condense during telophase I requiring them to re-condense in prophase II.[3]:100[10] If chromosomes do not need to re-condense, prophase II often proceeds very quickly as is seen in the model organism Arabidopsis.[10]
Prophase I arrest
Female mammals and birds are born possessing all the oocytes needed for future ovulations, and these oocytes are arrested at the prophase I stage of meiosis.[15] In humans, as an example, oocytes are formed between three and four months of gestation within the fetus and are therefore present at birth. During this prophase I arrested stage (dictyate), which may last for decades, four copies of the genome are present in the oocytes. The adaptive significance of prophase I arrest is still not fully understood. However, it has been proposed that the arrest of oocytes at the four genome copy stage may provide the informational redundancy needed to repair damage in the DNA of the germline.[15] The repair process used appears to be homologous recombinational repair[15][16] Prophase arrested oocytes have a high capability for efficient repair of DNA damages.[16] DNA repair capability appears to be a key quality control mechanism in the female germ line and a critical determinant of fertility.[16]
Differences in plant and animal cell prophase
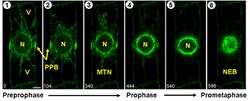
The most notable difference between prophase in plant cells and animal cells occurs because plant cells lack centrioles. The organization of the spindle apparatus is associated instead with foci at opposite poles of the cell or is mediated by chromosomes. Another notable difference is preprophase, an additional step in plant mitosis that results in formation of the preprophase band, a structure composed of microtubules. In mitotic prophase I of plants, this band disappears.[10]
Cell checkpoints
Prophase I in meiosis is the most complex iteration of prophase that occurs in both plant cells and animal cells.[3] To ensure pairing of homologous chromosomes and recombination of genetic material occurs properly, there are cellular checkpoints in place. The meiotic checkpoint network is a DNA damage response system that controls double strand break repair, chromatin structure, and the movement and pairing of chromosomes.[17] The system consists of multiple pathways (including the meiotic recombination checkpoint) that prevent the cell from entering metaphase I with errors due to recombination.[18]
See also
References
- ↑ 1.0 1.1 Thompson & Thompson Genetics in Medicine. Philadelphia: Elsevier. 2016. pp. 12–20. ISBN 9781437706963.
- ↑ 2.0 2.1 2.2 "Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy". Science 320 (5881): 1332–36. June 2008. doi:10.1126/science.1156947. PMID 18535242. Bibcode: 2008Sci...320.1332S.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 Genetics From Genes to Genomes. New York: McGraw-Hill. 2008. pp. 90–103. ISBN 978-0-07-284846-5. https://archive.org/details/genetics00lela_0/page/90.
- ↑ 4.0 4.1 4.2 Plant Cytogenetics (Third ed.). Boca Raton, FL: CBC Press, Taylor & Francis Group. 2017. pp. 19. ISBN 9781439884188.
- ↑ "G-banding in plant chromosomes". Genome 30: 48–51. 1988. doi:10.1139/g88-009.
- ↑ "High resolution bands in maize chromosomes by G-banding methods". Theoretical and Applied Genetics 80 (2): 265–72. August 1990. doi:10.1007/BF00224397. PMID 24220906.
- ↑ "Silver-stained structures in mammalian meiotic prophase". Chromosoma 70 (2): 195–203. January 1979. doi:10.1007/bf00288406. PMID 85512.
- ↑ "The nature and mechanisms of chromosome banding". Cancer Genetics and Cytogenetics 6 (1): 59–87. May 1982. doi:10.1016/0165-4608(82)90022-x. PMID 7049353.
- ↑ "Visualizing DNA domains and sequences by microscopy: a fifty-year history of molecular cytogenetics". Genome 46 (6): 943–6. December 2003. doi:10.1139/g03-107. PMID 14663510.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 Plant Physiology and Development. Sunderland MA: Sinauer Associates. 2015. pp. 35–39. ISBN 978-1-60535-255-8.
- ↑ 11.0 11.1 "Electron microscopic studies on the Silver-stained Nucleolar Cycle of Physarum Polycephalum". Acta Botanica Sinica 43 (7): 680–5. 2001. http://www.jipb.net/pubsoft/content/2/2071/X000541(PS2).pdf. Retrieved 24 February 2015.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Thompson & Thompson Genetics in Medicine. Philadelphia: Elsevier. 2016. pp. 12–20. ISBN 978-1-4377-0696-3.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Essential Cell Biology. New York NY: Garland Science. 2004. pp. 639–658. ISBN 978-0-8153-3481-1. https://archive.org/details/essentialcellbio00albe/page/639.
- ↑ "The leptotene-zygotene transition of meiosis". Annual Review of Genetics 32: 619–97. 1998. doi:10.1146/annurev.genet.32.1.619. PMID 9928494.
- ↑ 15.0 15.1 15.2 "Why is meiosis arrested?". Journal of Theoretical Biology 194 (2): 275–87. September 1998. doi:10.1006/jtbi.1998.0761. PMID 9778439. Bibcode: 1998JThBi.194..275M.
- ↑ 16.0 16.1 16.2 "Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health". Proceedings of the National Academy of Sciences of the United States of America 117 (21): 11513–11522. May 2020. doi:10.1073/pnas.2001124117. PMID 32381741. Bibcode: 2020PNAS..11711513S.
- ↑ "Checking your breaks: surveillance mechanisms of meiotic recombination". Current Biology 16 (6): R217-28. March 2006. doi:10.1016/j.cub.2006.03.009. PMID 16546077.
- ↑ "Checkpoint mechanisms: the puppet masters of meiotic prophase". Trends in Cell Biology 21 (7): 393–400. July 2011. doi:10.1016/j.tcb.2011.03.004. PMID 21531561.
External links
de:Mitose#Prophase
 |


