Biology:Excitatory amino acid transporter 2
 Generic protein structure example |
Excitatory amino acid transporter 2 (EAAT2) also known as solute carrier family 1 member 2 (SLC1A2) and glutamate transporter 1 (GLT-1) is a protein that in humans is encoded by the SLC1A2 gene.[1][2] Alternatively spliced transcript variants of this gene have been described, but their full-length nature is not known.[2]
Function
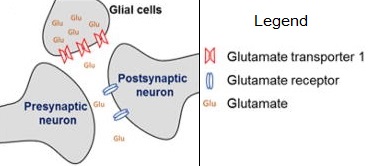
SLC1A2 / EAAT2 is a member of a family of the solute carrier family of proteins. The membrane-bound protein is the principal transporter that clears the excitatory neurotransmitter glutamate from the extracellular space at synapses in the central nervous system. Glutamate clearance is necessary for proper synaptic activation and to prevent neuronal damage from excessive activation of glutamate receptors.[2] EAAT2 is responsible for over 90% of glutamate reuptake within the brain.[3][4]
Clinical significance
Mutations in and decreased expression of this protein are associated with amyotrophic lateral sclerosis (ALS).[2] The drug riluzole approved for the treatment of ALS upregulates EAAT2.[5]
Ceftriaxone, an antibiotic, has been shown to induce/enhance the expression of EAAT2, resulting in reduced glutamate activity.[6] Ceftriaxone has been shown to reduce the development and expression of tolerance to opiates and other drugs of abuse. EAAT2 may possess an important role in drug addiction and tolerance to addictive drugs.[7]
Upregulation of EAAT2 (GLT-1) causes impairment of prepulse inhibition, a sensory gating deficit present in schizophrenics and schizophrenia animal models.[8][9] Some antipsychotics have been shown to reduce the expression of EAAT2.[10][11]
Interactions
SLC1A2 has been shown to interact with JUB.[12]
As a drug target
EAAT2/GLT-1, being the most abundant subtype of glutamate transporter in the CNS, plays a key role in regulation of glutamate neurotransmission. Dysfunction of EAAT2 has been correlated with various pathologies such as traumatic brain injury, stroke, Amyotrophic lateral sclerosis (ALS), Alzheimer's disease, among others. Therefore, activators of the function or enhancers of the expression of EAAT2/GLT-1 could serve as a potential therapy for these conditions. Translational activators of EAAT2/GLT-1, such as ceftriaxone and LDN/OSU-0212320, have been described to have significant protective effects in animal models of ALS and epilepsy. In addition, pharmacological activators of the activity of EAAT2/GLT-1 have been explored for decades and are currently emerging as promising tools for neuroprotection, having potential advantages over expression activators.[13]
DL-TBOA, WAY-213,613, and dihydrokainic acid are known inhibitors of the protein, and function as excitotoxins. They can be considered a novel class of nerve agent toxins, inducing toxic levels of glutamate through transport inhibition in a manner analogous to the effect of sarin on cholinesterase. Antidotes for such a poisoning have never been formally tested for efficacy and are not readily available for medical use.[14]
Addiction to certain drugs (e.g., cocaine, heroin, alcohol, and nicotine) is correlated with a persistent reduction in the expression of EAAT2 in the nucleus accumbens (NAcc);[15] the reduced expression of EAAT2 in this region is implicated in addictive drug-seeking behavior.[15] In particular, the long-term dysregulation of glutamate neurotransmission in the NAcc of addicts is associated with an increase in vulnerability to relapse after re-exposure to the addictive drug or its associated drug cues.[15] Drugs which help to normalize the expression of EAAT2 in this region, such as N-acetylcysteine, have been proposed as an adjunct therapy for the treatment of addiction to cocaine, nicotine, alcohol, and other drugs.[15]
See also
References
- ↑ "Cloning and expression of a rat brain L-glutamate transporter". Nature 360 (6403): 464–7. Dec 1992. doi:10.1038/360464a0. PMID 1448170. Bibcode: 1992Natur.360..464P.
- ↑ 2.0 2.1 2.2 2.3 "Entrez Gene: SLC1A2 solute carrier family 1 (glial high affinity glutamate transporter), member 2". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=6506.
- ↑ 3.0 3.1 "Designing Novel Nanoformulations Targeting Glutamate Transporter Excitatory Amino Acid Transporter 2: Implications in Treating Drug Addiction". J. Pers. Nanomed. 1 (1): 3–9. July 2015. PMID 26635971. "The glutamate transporter 1 (GLT1)/ excitatory amino acid transporter 2 (EAAT2) is responsible for the reuptake of more than 90% glutamate in the CNS [12–14].".
- ↑ Holmseth S; Scott HA; Real K; Lehre KP; Leergaard TB; Bjaalie JG; Danbolt NC (2009). "The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation". Neuroscience 162 (4): 1055–71. doi:10.1016/j.neuroscience.2009.03.048. PMID 19328838. "Since then, a family of five high-affinity glutamate transporters has been characterized that is responsible for the precise regulation of glutamate levels at both synaptic and extrasynaptic sites, although the glutamate transporter 1 (GLT1) is responsible for more than 90% of glutamate uptake in the brain.3 The importance of GLT1 is further highlighted by the large number of neuropsychiatric disorders associated with glutamate-induced neurotoxicity.
Clarification of nomenclature
The major glial glutamate transporter is referred to as GLT1 in the rodent literature and excitatory amino acid transporter 2 (EAAT2) in the human literature.". - ↑ "Riluzole elevates GLT-1 activity and levels in striatal astrocytes". Neurochem. Int. 60 (1): 31–8. 2012. doi:10.1016/j.neuint.2011.10.017. PMID 22080156.
- ↑ "Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes". J. Biol. Chem. 283 (19): 13116–23. May 2008. doi:10.1074/jbc.M707697200. PMID 18326497.
- ↑ "Using glutamate homeostasis as a target for treating addictive disorders". Behav Pharmacol 21 (5–6): 514–22. 2010. doi:10.1097/FBP.0b013e32833d41b2. PMID 20634691.
- ↑ "GLT-1 upregulation impairs prepulse inhibition of the startle reflex in adult rats". Glia 57 (7): 703–13. 2009. doi:10.1002/glia.20798. PMID 18985735.
- ↑ "The mGluR2/3 agonist LY379268 blocks the effects of GLT-1 upregulation on prepulse inhibition of the startle reflex in adult rats". Neuropsychopharmacology 35 (6): 1253–60. 2010. doi:10.1038/npp.2009.225. PMID 20072121.
- ↑ "Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain". Neurosci. Lett. 347 (2): 81–4. 2003. doi:10.1016/S0304-3940(03)00653-0. PMID 12873733.
- ↑ "Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures". Glia 50 (3): 276–9. 2005. doi:10.1002/glia.20172. PMID 15739191.
- ↑ "The amino terminus of the glial glutamate transporter GLT-1 interacts with the LIM protein Ajuba". Mol. Cell. Neurosci. 19 (2): 152–64. February 2002. doi:10.1006/mcne.2001.1066. PMID 11860269.
- ↑ "Current approaches to enhance glutamate transporter function and expression.". Journal of Neurochemistry 134 (6): 982–1007. June 20, 2015. doi:10.1111/jnc.13200. PMID 26096891. https://zenodo.org/record/889649.
- ↑ KEIKO SHIMAMOTO, BRUNO LEBRUN, YOSHIMI YASUDA-KAMATANI, MASAHIRO SAKAITANI, YASUSHI SHIGERI, NOBORU YUMOTO, and TERUMI NAKAJIMA (February 1998). "DL-threo-b-Benzyloxyaspartate, A Potent Blocker of Excitatory Amino Acid Transporters". Molecular Pharmacology 53 (2): 195–201. doi:10.1124/mol.53.2.195. PMID 9463476. http://molpharm.aspetjournals.org/content/molpharm/53/2/195.full.pdf.
- ↑ 15.0 15.1 15.2 15.3 "Potential role of N-acetylcysteine in the management of substance use disorders". CNS Drugs 28 (2): 95–106. 2014. doi:10.1007/s40263-014-0142-x. PMID 24442756.
Further reading
- "Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis". J. Neurovirol.. 10 10 (Suppl 1): 25–32. 2004. doi:10.1080/jnv.10.s1.25.32. PMID 14982736.
- "Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex". J. Neurosci. 14 (9): 5559–69. 1994. doi:10.1523/jneurosci.14-09-05559.1994. PMID 7521911.
- "Cloning and characterization of a glutamate transporter cDNA from human brain and pancreas". Biochim. Biophys. Acta 1195 (1): 185–8. 1994. doi:10.1016/0005-2736(94)90026-4. PMID 7522567.
- "Assignment of the gene SLC1A2 coding for the human glutamate transporter EAAT2 to human chromosome 11 bands p13-p12". Cytogenet. Cell Genet. 71 (3): 212–3. 1995. doi:10.1159/000134111. PMID 7587378.
- "Molecular cloning of human brain glutamate/aspartate transporter II". Biochim. Biophys. Acta 1191 (2): 393–6. 1994. doi:10.1016/0005-2736(94)90192-9. PMID 8172925.
- "A "double adaptor" method for improved shotgun library construction". Anal. Biochem. 236 (1): 107–13. 1996. doi:10.1006/abio.1996.0138. PMID 8619474.
- "Large-scale concatenation cDNA sequencing". Genome Res. 7 (4): 353–8. 1997. doi:10.1101/gr.7.4.353. PMID 9110174.
- "Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study". Brain Res. Mol. Brain Res. 52 (1): 17–31. 1997. doi:10.1016/S0169-328X(97)00233-7. PMID 9450673.
- "DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters". Mol. Pharmacol. 53 (2): 195–201. 1998. doi:10.1124/mol.53.2.195. PMID 9463476.
- "Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis". Neuron 20 (3): 589–602. 1998. doi:10.1016/S0896-6273(00)80997-6. PMID 9539131.
- "Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis". Ann. Neurol. 43 (5): 645–53. 1998. doi:10.1002/ana.410430514. PMID 9585360.
- "Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity". J. Biol. Chem. 276 (1): 576–82. 2001. doi:10.1074/jbc.M003779200. PMID 11031254.
- "Differential RNA cleavage and polyadenylation of the glutamate transporter EAAT2 in the human brain". Brain Res. Mol. Brain Res. 80 (2): 244–51. 2000. doi:10.1016/S0169-328X(00)00139-X. PMID 11038258.
- "Glutamate transporter EAAT2 splice variants occur not only in ALS, but also in AD and controls". Neurology 55 (8): 1082–8. 2000. doi:10.1212/wnl.55.8.1082. PMID 11071482.
- "Intron 7 retention and exon 9 skipping EAAT2 mRNA variants are not associated with amyotrophic lateral sclerosis". Ann. Neurol. 49 (5): 643–9. 2001. doi:10.1002/ana.1029. PMID 11357955.
- "Role of glutamate transporters in the regulation of glutathione levels in human macrophages". Am. J. Physiol., Cell Physiol. 281 (6): C1964-70. 2001. doi:10.1152/ajpcell.2001.281.6.C1964. PMID 11698255.
- "Role of glial glutamate transporters in the facilitatory action of FK960 on hippocampal neurotransmission". Brain Res. Mol. Brain Res. 97 (1): 7–12. 2001. doi:10.1016/S0169-328X(01)00304-7. PMID 11744157.
- "Benzodiazepines differently modulate EAAT1/GLAST and EAAT2/GLT1 glutamate transporters expressed in CHO cells". Neurochem. Int. 40 (4): 321–6. 2002. doi:10.1016/S0197-0186(01)00087-0. PMID 11792462.
- "The amino terminus of the glial glutamate transporter GLT-1 interacts with the LIM protein Ajuba". Mol. Cell. Neurosci. 19 (2): 152–64. 2002. doi:10.1006/mcne.2001.1066. PMID 11860269.
- "Distribution of two splice variants of the glutamate transporter GLT1 in the retinas of humans, monkeys, rabbits, rats, cats, and chickens". J. Comp. Neurol. 445 (1): 1–12. 2002. doi:10.1002/cne.10095. PMID 11891650.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |