Biology:Luciferin

Luciferin (from la lucifer 'light-bearer') is a generic term for the light-emitting compound found in organisms that generate bioluminescence. Luciferins typically undergo an enzyme-catalyzed reaction with molecular oxygen. The resulting transformation, which usually involves breaking off a molecular fragment, produces an excited state intermediate that emits light upon decaying to its ground state. The term may refer to molecules that are substrates for both luciferases and photoproteins.[1]
Types
Luciferins are a class of small-molecule substrates that react with oxygen in the presence of a luciferase (an enzyme) to release energy in the form of light. It is not known just how many types of luciferins there are, but some of the better-studied compounds are listed below.
Because of the chemical diversity of luciferins, there is no clear unifying mechanism of action, except that all require molecular oxygen,[2] The variety of luciferins and luciferases, their diverse reaction mechanisms and the scattered phylogenetic distribution indicate that many of them have arisen independently in the course of evolution.[2]
Firefly
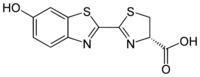
Firefly luciferin is the luciferin found in many Lampyridae species, such as P. pyralis. It is the substrate of beetle luciferases (EC 1.13.12.7) responsible for the characteristic yellow light emission from fireflies, though can cross-react to produce light with related enzymes from non-luminous species.[3] The chemistry is unusual, as adenosine triphosphate (ATP) is required for light emission, in addition to molecular oxygen.[4]
Snail

Latia luciferin is, in terms of chemistry, (E)-2-methyl-4-(2,6,6-trimethyl-1-cyclohex-1-yl)-1-buten-1-ol formate and is from the freshwater snail Latia neritoides.[5]
Bacterial

Bacterial luciferin is two-component system consisting of flavin mononucleotide and a fatty aldehyde found in bioluminescent bacteria.[6]
Coelenterazine
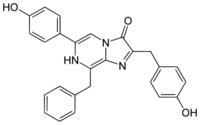
Coelenterazine is found in radiolarians, ctenophores, cnidarians, squid, brittle stars, copepods, chaetognaths, fish, and shrimp. It is the prosthetic group in the protein aequorin responsible for the blue light emission.[7]
Dinoflagellate

Dinoflagellate luciferin is a chlorophyll derivative (i. e. a tetrapyrrole) and is found in some dinoflagellates, which are often responsible for the phenomenon of nighttime glowing waves (historically this was called phosphorescence, but is a misleading term). A very similar type of luciferin is found in some types of euphausiid shrimp.[8]
Vargulin
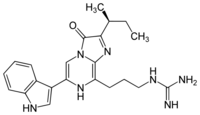
Vargulin is found in certain ostracods and deep-sea fish, to be specific, Poricthys. Like the compound coelenterazine, it is an imidazopyrazinone and emits primarily blue light in the animals.
Fungi

Foxfire is the bioluminescence created by some species of fungi present in decaying wood. While there may be multiple different luciferins within the kingdom of fungi, 3-hydroxy hispidin was determined to be the luciferin in the fruiting bodies of several species of fungi, including Neonothopanus nambi, Omphalotus olearius, Omphalotus nidiformis, and Panellus stipticus.[9]
Usage in science
Luciferin is widely used in science and medicine as a method of in vivo imaging, using living organisms to non-invasively detect images and in molecular imaging. The reaction between luciferin substrate paired with the receptor enzyme luciferase produces a catalytic reaction, generating bioluminescence.[10] This reaction and the luminescence produced is useful for imaging such as detecting tumors from cancer or capable of measuring gene expression.
References
- ↑ "Chemistries and colors of bioluminescent reactions: a review". Gene 173 (1 Spec No): 5–11. 1996. doi:10.1016/0378-1119(95)00676-1. PMID 8707056.
- ↑ 2.0 2.1 "Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems". Journal of Molecular Evolution 19 (5): 309–321. 1983. doi:10.1007/BF02101634. PMID 6358519. Bibcode: 1983JMolE..19..309H.
- ↑ "Larval Tenebrio molitor (Coleoptera: Tenebrionidae) Fat Body Extracts Catalyze Firefly D-Luciferin-and ATP-Dependent Chemiluminescence: A Luciferase-like Enzyme". Photochemistry and Photobiology 63 (6): 713–718. 1996. doi:10.1111/j.1751-1097.1996.tb09620.x.
- ↑ "Function of adenosine triphosphate in the activation of luciferin". Archives of Biochemistry and Biophysics 64 (2): 257–271. October 1956. doi:10.1016/0003-9861(56)90268-5. PMID 13363432.
- ↑ EC 1.14.99.21. ORENZA: a database of ORphan ENZyme Activities, accessed 27 November 2009.
- ↑ "Bacterial illumination". Bioscience Explained 1 (1). 2001. https://medarbetarportalen.gu.se/digitalAssets/1566/1566430_photoen.pdf.
- ↑ "Chemical nature of bioluminescence systems in coelenterates". Proceedings of the National Academy of Sciences of the United States of America 72 (4): 1546–1549. April 1975. doi:10.1073/pnas.72.4.1546. PMID 236561. Bibcode: 1975PNAS...72.1546S.
- ↑ "Crossreactivity between the light-emitting systems of distantly related organisms: Novel type of light-emitting compound". Proceedings of the National Academy of Sciences of the United States of America 77 (3): 1394–1397. March 1980. doi:10.1073/pnas.77.3.1394. PMID 16592787. Bibcode: 1980PNAS...77.1394D.
- ↑ "The Chemical Basis of Fungal Bioluminescence". Angewandte Chemie 54 (28): 8124–8128. July 2015. doi:10.1002/anie.201501779. PMID 26094784. Bibcode: 2015ACIE...54.8124P.
- ↑ "Bioluminescence imaging: progress and applications". Trends in Biotechnology 29 (12): 624–633. December 2011. doi:10.1016/j.tibtech.2011.06.010. PMID 21788092.
External links
- "Major luciferin types". The Bioluminescence Web Page. University of California, Santa Barbara. 2009-01-09. http://www.lifesci.ucsb.edu/~biolum/chem/detail1.html.
 |
