Biology:Neuroglobin
| Neuroglobin | |
|---|---|
 | |
| Identifiers | |
| Symbol | NGB |
| NCBI gene | 58157 |
| HGNC | 6553 |
| OMIM | 605304 |
| UniProt | Q9NPG2 |
| Other data | |
| Locus | Chr. 14 q24 |
Neuroglobin is a member of the vertebrate globin family involved in cellular oxygen homeostasis and reactive oxygen/nitrogen scavenging. It is an intracellular hemoprotein expressed in the central and peripheral nervous system, cerebrospinal fluid, retina and endocrine tissues. Neuroglobin is a monomer that reversibly binds oxygen with an affinity higher than that of hemoglobin. It also increases oxygen availability to brain tissue and provides protection under hypoxic or ischemic conditions, potentially limiting brain damage. Neuroglobin were in the past found only in vertebrate neurons, but recently in 2013, were found in the neurons of unrelated protostomes, like photosynthetic acoel as well as radiata such as jellyfish. In addition to neurons, neuroglobin is present in astrocytes in certain pathologies of the rodent brain[1][2] and in the physiological seal brain.[3] This is thought to be due to convergent evolution.[4] It is of ancient evolutionary origin, and is homologous to nerve globins of invertebrates. Recent research confirmed the presence of human neuroglobin protein in cerebrospinal fluid (CSF).[5]
Neuroglobin was first identified by Thorsten Burmester et al. in 2000.[6]
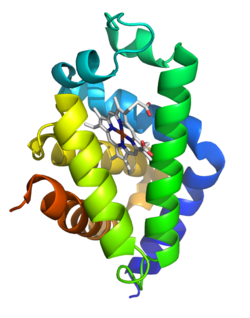
The 3D structure of human neuroglobin was determined in 2003.[7] The next year, murine neuroglobin was determined at a higher resolution.[8]
A practical treatment for carbon monoxide poisoning based on binding of CO by neuroglobin (Ngb) with a mutated distal histidine (H64Q) appears to be possible.[9]
See also
References
- ↑ "In vivo expression of neuroglobin in reactive astrocytes during neuropathology in murine models of traumatic brain injury, cerebral malaria, and autoimmune encephalitis". Glia 58 (10): 1220–7. August 2010. doi:10.1002/glia.21002. PMID 20544857.
- ↑ "17β-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor β-mediated neuroglobin up-regulation". Journal of Neuroendocrinology 25 (3): 260–70. March 2013. doi:10.1111/jne.12007. PMID 23190172. https://digital.csic.es/bitstream/10261/73308/1/accesoRestringido.pdf.
- ↑ "When the brain goes diving: glial oxidative metabolism may confer hypoxia tolerance to the seal brain". Neuroscience 163 (2): 552–60. October 2009. doi:10.1016/j.neuroscience.2009.06.058. PMID 19576963.
- ↑ science.gov, Neuroglobins, Pivotal Proteins Associated with Emerging Neural Systems and Precursors of Metazoan Globin Diversity by Lechauve, Christophe; Jager, Muriel; Laguerre, Laurent; Kiger, Laurent; Correc, Gaelle; Leroux, Cedric; Vinogradov, Serge; Czjzek, Mirjam; Marden, Michael C.; Bail
- ↑ "Human neuroglobin protein in cerebrospinal fluid". Proteome Science 3 (1): 2. February 2005. doi:10.1186/1477-5956-3-2. PMID 15730566. PMC 554085. https://deepblue.lib.umich.edu/bitstream/2027.42/112428/1/12953_2004_Article_18.pdf.
- ↑ "A vertebrate globin expressed in the brain". Nature 407 (6803): 520–3. September 2000. doi:10.1038/35035093. PMID 11029004. Bibcode: 2000Natur.407..520B.
- ↑ Alessandra Pesce; Sylvia Dewilde; Marco Nardini; Luc Moens; Paolo Ascenzi; Thomas Hankeln; Thorsten Burmester; Martino Bolognes (2003). "Human Brain Neuroglobin Structure Reveals a Distinct Mode of Controlling Oxygen Affinity". Structure 11 (9): 1087–1095. doi:10.1016/S0969-2126(03)00166-7. PMID 12962627.
- ↑ Beatrice Vallone; Karin Nienhaus; Maurizio Brunori; G. Ulrich Nienhaus (2004). "The structure of murine neuroglobin: Novel pathways for ligand migration and binding". Proteins: Structure, Function, and Bioinformatics 56 (1): 85–92. doi:10.1002/prot.20113. PMID 15162488.
- ↑ Rydzewski, J; Nowak, W (2018). "Photoinduced transport in an H64Q neuroglobin antidote for carbon monoxide poisoning". The Journal of Chemical Physics 148 (11): 115101. doi:10.1063/1.5013659. PMID 29566507. Bibcode: 2018JChPh.148k5101R.
External links
- neuroglobin at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
