Biology:Plant defensin
| Plant defensin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Plant defensin | ||||||||
| Pfam | PF00304 | ||||||||
| Pfam clan | CL0054 | ||||||||
| InterPro | IPR008176 | ||||||||
| PROSITE | PDOC00725 | ||||||||
| SCOP2 | 1gps / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1jkz | ||||||||
| CDD | cd00107 | ||||||||
| |||||||||
| Plant defensin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | Plant defensin | ||||||||
| Pfam | PF00304 | ||||||||
| Pfam clan | CL0054 | ||||||||
| InterPro | IPR008176 | ||||||||
| PROSITE | PDOC00725 | ||||||||
| SCOP2 | 1gps / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1jkz | ||||||||
| CDD | cd00107 | ||||||||
| |||||||||
Plant defensins (formerly gamma-thionins) are a family of primitive, highly stable, cysteine-rich defensins found in plants that function to defend them against pathogens and parasites.[1] Defensins are integral components of the innate immune system and belong to the ancient superfamily of antimicrobial peptides (AMPs). AMPs are also known as host defense peptides (HDPs),[2] and they are thought to have diverged about 1.4 billion years ago before the evolution of prokaryotes and eukaryotes.[3][4] They are ubiquitous in almost all plant species, functionally diverse, and their primary structure varies significantly from one species to the next, except for a few cysteine residues, which stabilize the protein structure through disulfide bond formation.[1] Plant defensins usually have a net positive charge due to the abundance of cationic amino acids[5] and are generally divided into two classes. Those in the class II category contain a C-terminal pro-peptide domain of approximately 33 amino acids[5] and are targeted to the vacuole,[6] while the class I defensins lack this domain and mature in the cell wall. Unlike their class I counterparts, class II plant defensins are relatively smaller, and their acidic C-terminal prodomain is hypothesized to contribute to their vacuolar targeting.[7] The first plant defensins were discovered in barley and wheat in 1990 and were initially designated as γ-thionins.[8][9] In 1995, the name was changed to 'plant defensin' when it was identified that they are evolutionarily unrelated to other thionins and were more similar to defensins from insects and mammals.[10][11]
Tissue-specific localization
A large number of defensins were initially isolated from seeds, where they are linked to the defense of germinating seeds against fungal pathogens,[11] but recent advances in bioinformatics and molecular biology techniques have revealed that these peptides are present in other parts of the plant, including flowers and roots.[12][3] Defensins can be expressed in two ways: constitutively or induced under certain stresses. For example, the defensin AtPDF2.2 from Arabidopsis thaliana is expressed constitutively,[13] while another defensin from the same plant is induced by methyl jasmonate and ethylene.[14]
Structure and evolution
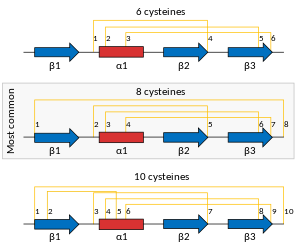
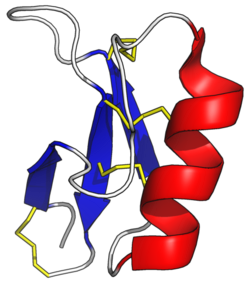
Plant defensins are members of the protein superfamily called the cis-defensins or CSαβ fold.[15] This superfamily includes arthropod defensins and fungal defensins (but not defensins found in mammals). It also includes several families of proteins not involved in the immune system, including plant S-locus 11 proteins involved in self-incompatibility during reproduction and toxin proteins in scorpion venoms.[16][17] Defensin proteins are produced as an amphipathic protein precursor with one or two pro-domains that are removed to make the final mature protein. In their mature form, they generally consist of about 45 to 50 amino acid residues. The folded globular structure is characterized by a well-defined 3-stranded anti-parallel beta-sheet and a short alpha-helix.[18] The structure of most plant defensins is cross-linked by four disulfide bridges: three in the core and one linking the N- and C-termini. Some plant defensins have only the core three disulfides, and a few have been found with an additional one (resulting in five total bridges).[19] Two of these bonds, those formed between the α-helix and the last β-strand, are arranged into the Cys-stabilized α-helix β-strand (CSαβ) motif, which play significant roles in their biological activities and stability.[20][21] The globular structures of plant defensins make them resistant to degradation by proteolytic digestion and stable up to a pH and a temperature range of 10 and 90 degrees Celsius, respectively.[22][23]
Functions
Plant defensins are a large component of the plant innate immune system. They are regarded as highly promiscuous molecules due to their diverse biological functions. A plant genome typically contains large numbers of different defensin genes[24] that vary in their efficacy against different pathogens and the amount they are expressed in different tissues.[25] In addition to their functions in the immune system, many of these low-molecular-weight peptides have developed additional roles in aiding reproduction and abiotic stress tolerance.[1]
Antimicrobial activity
Plant defensins elicit diverse antimicrobial properties, including antibacterial,[2] and antifungal[26] activities. The modes of action of different defensins depend on the type of organism and specific molecular targets,[27][2] although their exact mechanisms of action vary. For instance, their antifungal activities, which are their best-characterized property, are attributed to their ability to interact with lipid structures on pathogenic fungi surfaces. These include sphingolipids,[28] glucosyceramide,[29] and phosphatidic acid[30] Apart from their capacity to attack and damage fungal membranes, these peptides have also been extensively researched for their capacity to trigger apoptosis and target other intracellular structures and biomolecules.[31] Plant defensins can spread their lethality by interfering with important developmental and/or regulatory processes, such as the cell cycle, when they perturb or disrupt the membrane of the fungus they target.[32] On the other hand, their ability to induce apoptosis has been linked to the bioaccumulation of reactive oxygen species[33] and the recruitment of specific caspases and caspase-associated proteins/[34] In mediating their antibacterial mechanisms, plant defensin has been shown to cause loss of cell viability by inducing an unfavorable morphological change in the bacterial target via membrane targeting and permeation.[35] This defensin-membrane interaction has been linked to the presence of the cationic amino acid residues arginine, lysine, and histidine.[36] Furthermore, studies have shown that plant defensin inhibits in vitro protein synthesis in a cell-free system,[37] and their interactions with the DNA of bacterial pathogens have also been documented, hinting that they might have a lethal effect on DNA replication or transcription.[35]
Enzyme inhibition
Some plant defensins have also been identified as enzyme inhibitors of α-amylase or trypsin.[38][39][40] It is believed that these are antifeedant activities to deter insects.[39] Typically, molecular modeling analysis of defensin expressed in Vigna unguiculata revealed that defensin inhibits α-amylase in the weevils Acanthoscelides obtectus and Zabrotes subfasciatus by binding via its N-terminal to the active site of the enzyme.[38] Defensins with alpha-amylase-inhibitory activity have also been identified in Sorghum bicolor,[39][41] suggesting defensins might interfere with carbohydrate metabolism in insect targets. Beyond their ability to inhibit alpha-amylases, defensins also demonstrate inhibitory properties toward trypsin and chymotrypsin. For instance, two defensins from the seeds of Cassia fistula have been documented to inhibit the activity of trypsin,[42][40] and Capsicum annuum (CanDef-20) defensin has been reported to alter insect metabolism and retard growth in a number of ways, such as upregulation of lipase, serine endopeptidase, glutathione S-transferase, cadherin, alkaline phosphatase, and aminopeptidases and triggering transposon mobilization in Helicoverpa armigera.[43]
Anti-cancer
An additional promiscuous activity of some plant defensins is stopping the growth or disrupting the membranes of cancer cells in in vitro experiments.[44][45] This interaction is basically facilitated and made stable due to the negatively charged membrane components on cancer cells relative to the positive charge of defensin.[46][47] Typically, in addition to reducing the viability of melanoma and leukemia cells, Nicotiana alata defensin 1 (NaD1) reportedly induces the death of tumor cells within 30 minutes of contact.[48] This necrotic-like cell killing was facilitated by the binding of NaD1 to the plasma membrane lipid, phosphatidylinositol 4,5-bisphosphate (PIP2), which resulted in subsequent cell lysis. Defensins from plant origins have also shown potent toxicity towards colon and breast cancer.[49]
Abiotic stress tolerance
Plant defensins are expressed in diverse organelles and tissues in plants, and exposure of plants to specific environmental stresses has been associated with increased expression of defensin, suggesting their function in abiotic stress defense.[50] By means of endoplasmic reticulum adaptive activity, plant defensins AhPDF1.1 and AhPDF1.2 were recently found to exhibit metal (Zn) tolerance in yeast and plants.[51] Also, a defensin from paddy has been documented to sequester cadmium in rice, preventing its intracellular distribution.[7] Overexpression of chickpea defensin gene also confers tolerance to water-deficit stress in Arabidopsis thaliana.[52]
Examples
The following plant proteins belong to this family:
- The flower-specific Nicotiana alata defensin (NaD1)
- Gamma-thionins from Triticum aestivum (wheat) endosperm (gamma-purothionins) and gamma-hordothionins from Hordeum vulgare (barley) are toxic to animal cells and inhibit protein synthesis in cell free systems.[18]
- A flower-specific thionin (FST) from Nicotiana tabacum (common tobacco).[53]
- Antifungal proteins (AFP) from the seeds of Brassicaceae species such as radish, mustard, turnip and Arabidopsis thaliana (thale cress).[54]
- Inhibitors of insect alpha-amylases from sorghum.[41]
- Probable protease inhibitor P322 from Solanum tuberosum (potato).
- A germination-related protein from Vigna unguiculata (cowpea).[55]
- Anther-specific protein SF18 from sunflower. SF18 is a protein that contains a gamma-thionin domain at its N-terminus and a proline-rich C-terminal domain.
- Glycine max (soybean) sulfur-rich protein SE60.[56]
- Vicia faba (broad bean) antibacterial peptides fabatin-1 and -2.
Databases
A database for antimicrobial peptides, including defensins is available: PhytAMP (http://phytamp.hammamilab.org).[57]
References
- ↑ 1.0 1.1 1.2 "The evolution, function and mechanisms of action for plant defensins". Seminars in Cell & Developmental Biology 88: 107–118. April 2019. doi:10.1016/j.semcdb.2018.02.004. PMID 29432955. https://figshare.com/articles/journal_contribution/13213427.
- ↑ 2.0 2.1 2.2 "Antibacterial Activity of Plant Defensins". Molecular Plant-Microbe Interactions 32 (5): 507–514. May 2019. doi:10.1094/mpmi-08-18-0229-cr. PMID 30501455.
- ↑ 3.0 3.1 "Plant defensins and defensin-like peptides - biological activities and biotechnological applications". Current Pharmaceutical Design 17 (38): 4270–4293. December 2011. doi:10.2174/138161211798999447. PMID 22204427.
- ↑ "Morphological and ecological complexity in early eukaryotic ecosystems". Nature 412 (6842): 66–69. July 2001. doi:10.1038/35083562. PMID 11452306.
- ↑ 5.0 5.1 "Biotechnological potential of antimicrobial peptides from flowers". Peptides 29 (10): 1842–1851. October 2008. doi:10.1016/j.peptides.2008.06.003. PMID 18602431. https://repositorio.ucb.br:9443/jspui/handle/123456789/7781.
- ↑ "The C-terminal propeptide of a plant defensin confers cytoprotective and subcellular targeting functions". BMC Plant Biology 14 (1): 41. February 2014. doi:10.1186/1471-2229-14-41. PMID 24495600.
- ↑ 7.0 7.1 "Analysis of structures, functions, and transgenicity of phytopeptides defensin and thionin: a review". Beni-Suef University Journal of Basic and Applied Sciences 10 (1). 2021-01-14. doi:10.1186/s43088-020-00093-5. ISSN 2314-8543.
- ↑ "Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, gamma-hordothionin, from barley endosperm". European Journal of Biochemistry 194 (2): 533–539. December 1990. doi:10.1111/j.1432-1033.1990.tb15649.x. PMID 2176600.
- ↑ "gamma-Purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm". FEBS Letters 270 (1–2): 191–194. September 1990. doi:10.1016/0014-5793(90)81265-p. PMID 2226781.
- ↑ "Plant defensins: novel antimicrobial peptides as components of the host defense system". Plant Physiology 108 (4): 1353–1358. August 1995. doi:10.1104/pp.108.4.1353. PMID 7659744.
- ↑ 11.0 11.1 "Small cysteine-rich antifungal proteins from radish: their role in host defense". The Plant Cell 7 (5): 573–588. May 1995. doi:10.1105/tpc.7.5.573. PMID 7780308.
- ↑ "Plant defensins--prospects for the biological functions and biotechnological properties". Peptides 30 (5): 1007–1020. May 2009. doi:10.1016/j.peptides.2009.01.018. PMID 19428780.
- ↑ "The promoter of a plant defensin gene directs specific expression in nematode-induced syncytia in Arabidopsis roots". Plant Physiology and Biochemistry 49 (10): 1100–1107. October 2011. doi:10.1016/j.plaphy.2011.07.005. PMID 21813283.
- ↑ "Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway". The Plant Cell 8 (12): 2309–2323. December 1996. doi:10.1105/tpc.8.12.2309. PMID 8989885.
- ↑ "A Centipede Toxin Family Defines an Ancient Class of CSαβ Defensins". Structure 27 (2): 315–326.e7. February 2019. doi:10.1016/J.STR.2018.10.022. PMID 30554841. https://figshare.com/articles/journal_contribution/A_Centipede_Toxin_Family_Defines_an_Ancient_Class_of_CS_Defensins/13213265/1/files/25443938.pdf.
- ↑ "The Defensins Consist of Two Independent, Convergent Protein Superfamilies". Molecular Biology and Evolution 33 (9): 2345–2356. September 2016. doi:10.1093/molbev/msw106. PMID 27297472. https://figshare.com/articles/journal_contribution/The_defensins_consist_of_two_independent_convergent_protein_superfamilies/13211594/2/files/25442216.pdf.
- ↑ "Convergent evolution of defensin sequence, structure and function". Cellular and Molecular Life Sciences 74 (4): 663–682. February 2017. doi:10.1007/s00018-016-2344-5. PMID 27557668. https://figshare.com/articles/journal_contribution/13211600.
- ↑ 18.0 18.1 "Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: a structural motif common to toxic arthropod proteins". Biochemistry 32 (2): 715–724. January 1993. doi:10.1021/bi00053a041. PMID 8380707.
- ↑ "Structure of Petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds". Biochemistry 42 (27): 8214–8222. July 2003. doi:10.1021/bi034379o. PMID 12846570.
- ↑ "Refined three-dimensional solution structure of insect defensin A". Structure 3 (5): 435–448. May 1995. doi:10.1016/s0969-2126(01)00177-0. PMID 7663941.
- ↑ "Three-dimensional structure of natural charybdotoxin in aqueous solution by 1H-NMR. Charybdotoxin possesses a structural motif found in other scorpion toxins". European Journal of Biochemistry 196 (1): 19–28. February 1991. doi:10.1111/j.1432-1033.1991.tb15780.x. PMID 1705886.
- ↑ "Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase". Peptides 26 (7): 1120–1126. July 2005. doi:10.1016/j.peptides.2005.01.003. PMID 15949629.
- ↑ "Vulgarinin, a broad-spectrum antifungal peptide from haricot beans (Phaseolus vulgaris)". The International Journal of Biochemistry & Cell Biology 37 (8): 1626–1632. August 2005. doi:10.1016/j.biocel.2005.02.022. PMID 15896669.
- ↑ "Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants". The Plant Journal 51 (2): 262–280. July 2007. doi:10.1111/j.1365-313x.2007.03136.x. PMID 17565583.
- ↑ "Defensins--components of the innate immune system in plants". Current Protein & Peptide Science 6 (1): 85–101. February 2005. doi:10.2174/1389203053027575. PMID 15638771.
- ↑ "The antifungal activity of RsAFP2, a plant defensin from raphanus sativus, involves the induction of reactive oxygen species in Candida albicans". Journal of Molecular Microbiology and Biotechnology 13 (4): 243–247. 2007. doi:10.1159/000104753. PMID 17827975.
- ↑ "Antifungal plant defensins: increased insight in their mode of action as a basis for their use to combat fungal infections". Future Microbiology 12 (5): 441–454. April 2017. doi:10.2217/fmb-2016-0181. PMID 28339295.
- ↑ "DmAMP1, an antifungal plant defensin from dahlia (Dahlia merckii), interacts with sphingolipids from Saccharomyces cerevisiae". FEMS Microbiology Letters 226 (1): 169–173. September 2003. doi:10.1016/S0378-1097(03)00590-1. PMID 13129623.
- ↑ "Interactions of antifungal plant defensins with fungal membrane components". Peptides. Antimicrobial Peptides II 24 (11): 1705–1712. November 2003. doi:10.1016/j.peptides.2003.09.014. PMID 15019201.
- ↑ "Binding of phosphatidic acid by NsD7 mediates the formation of helical defensin-lipid oligomeric assemblies and membrane permeabilization". Proceedings of the National Academy of Sciences of the United States of America 113 (40): 11202–11207. October 2016. doi:10.1073/pnas.1607855113. PMID 27647905. Bibcode: 2016PNAS..11311202K.
- ↑ "Antibiotic activities of host defense peptides: more to it than lipid bilayer perturbation". Natural Product Reports 28 (8): 1350–1358. August 2011. doi:10.1039/c1np00022e. PMID 21617811.
- ↑ "Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle". Biochemistry 46 (4): 987–996. January 2007. doi:10.1021/bi061441j. PMID 17240982.
- ↑ "The Antifungal Plant Defensin HsAFP1 from Heuchera sanguinea Induces Apoptosis in Candida albicans". Frontiers in Microbiology 2: 47. 2011. doi:10.3389/fmicb.2011.00047. PMID 21993350.
- ↑ "The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans". Molecular Microbiology 84 (1): 166–180. April 2012. doi:10.1111/j.1365-2958.2012.08017.x. PMID 22384976.
- ↑ 35.0 35.1 "Modes of Action of a Bi-domain Plant Defensin MtDef5 Against a Bacterial Pathogen Xanthomonas campestris". Frontiers in Microbiology 9: 934. 2018-05-16. doi:10.3389/fmicb.2018.00934. PMID 29867843.
- ↑ "The Antifungal Plant Defensin HsAFP1 Is a Phosphatidic Acid-Interacting Peptide Inducing Membrane Permeabilization". Frontiers in Microbiology 8: 2295. 2017-11-21. doi:10.3389/fmicb.2017.02295. PMID 29209301.
- ↑ "Cloning and characterization of a plant defensin VaD1 from azuki bean". Journal of Agricultural and Food Chemistry 53 (4): 982–988. February 2005. doi:10.1021/jf0402227. PMID 15713009.
- ↑ 38.0 38.1 "Novel insights on the mechanism of action of alpha-amylase inhibitors from the plant defensin family". Proteins 73 (3): 719–729. November 2008. doi:10.1002/prot.22086. PMID 18498107.
- ↑ 39.0 39.1 39.2 "Plant alpha-amylase inhibitors and their interaction with insect alpha-amylases". European Journal of Biochemistry 269 (2): 397–412. January 2002. doi:10.1046/j.0014-2956.2001.02656.x. PMID 11856298.
- ↑ 40.0 40.1 "Plant gamma-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins". The International Journal of Biochemistry & Cell Biology 37 (11): 2239–2253. November 2005. doi:10.1016/j.biocel.2005.06.011. PMID 16084753.
- ↑ 41.0 41.1 "A new family of small (5 kDa) protein inhibitors of insect alpha-amylases from seeds or sorghum (Sorghum bicolar (L) Moench) have sequence homologies with wheat gamma-purothionins". FEBS Letters 279 (1): 101–104. February 1991. doi:10.1016/0014-5793(91)80261-z. PMID 1995329.
- ↑ "Defense proteins from seed of Cassia fistula include a lipid transfer protein homologue and a protease inhibitory plant defensin". Plant Science 159 (2): 243–255. November 2000. doi:10.1016/s0168-9452(00)00348-4. PMID 11074277.
- ↑ "Novel insights into plant defensin ingestion induced metabolic responses in the polyphagous insect pest Helicoverpa armigera". Scientific Reports 13 (1): 3151. February 2023. doi:10.1038/s41598-023-29250-3. PMID 36823197. Bibcode: 2023NatSR..13.3151M.
- ↑ "Phosphoinositide-mediated oligomerization of a defensin induces cell lysis". eLife 3: e01808. April 2014. doi:10.7554/ELIFE.01808. PMID 24692446.
- ↑ "The Tomato Defensin TPP3 Binds Phosphatidylinositol (4,5)-Bisphosphate via a Conserved Dimeric Cationic Grip Conformation To Mediate Cell Lysis". Molecular and Cellular Biology 35 (11): 1964–1978. June 2015. doi:10.1128/mcb.00282-15. PMID 25802281.
- ↑ "Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice". Clinical Cancer Research 11 (4): 1551–1562. February 2005. doi:10.1158/1078-0432.ccr-04-1645. PMID 15746060.
- ↑ "Mucins in cancer: function, prognosis and therapy". Nature Reviews. Cancer 9 (12): 874–885. December 2009. doi:10.1038/nrc2761. PMID 19935676.
- ↑ "The plant defensin NaD1 induces tumor cell death via a non-apoptotic, membranolytic process". Cell Death Discovery 3 (1): 16102. 2017-01-23. doi:10.1038/cddiscovery.2016.102. PMID 28179997.
- ↑ "Plant antimicrobial peptides as potential anticancer agents". BioMed Research International 2015: 735087. 2015. doi:10.1155/2015/735087. PMID 25815333.
- ↑ "Four plant defensins from an indigenous South African Brassicaceae species display divergent activities against two test pathogens despite high sequence similarity in the encoding genes". BMC Research Notes 4 (1): 459. October 2011. doi:10.1186/1756-0500-4-459. PMID 22032337.
- ↑ "The antifungal plant defensin AhPDF1.1b is a beneficial factor involved in adaptive response to zinc overload when it is expressed in yeast cells". MicrobiologyOpen 4 (3): 409–422. June 2015. doi:10.1002/mbo3.248. PMID 25755096.
- ↑ "Overexpression of Chickpea Defensin Gene Confers Tolerance to Water-Deficit Stress in Arabidopsis thaliana". Frontiers in Plant Science 10: 290. 2019-03-12. doi:10.3389/fpls.2019.00290. PMID 30915095.
- ↑ "A flower-specific cDNA encoding a novel thionin in tobacco". Molecular & General Genetics 234 (1): 89–96. July 1992. doi:10.1007/BF00272349. PMID 1495489.
- ↑ "A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species". FEBS Letters 316 (3): 233–240. February 1993. doi:10.1016/0014-5793(93)81299-F. PMID 8422949.
- ↑ "Stored mRNA in cotyledons of Vigna unguiculata seeds: nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination". Plant Molecular Biology 15 (1): 59–64. July 1990. doi:10.1007/BF00017724. PMID 2103443.
- ↑ "Nucleotide sequence of a cDNA encoding a low molecular weight sulfur-rich protein in soybean seeds". Plant Physiology 101 (2): 699–700. February 1993. doi:10.1104/pp.101.2.699. PMID 8278516.
- ↑ "PhytAMP: a database dedicated to antimicrobial plant peptides". Nucleic Acids Research 37 (Database issue): D963–D968. January 2009. doi:10.1093/nar/gkn655. PMID 18836196.
Subfamilies
 |