Biology:SDHA
 Generic protein structure example |
Succinate dehydrogenase complex, subunit A, flavoprotein variant is a protein that in humans is encoded by the SDHA gene.[1] This gene encodes a major catalytic subunit of succinate-ubiquinone oxidoreductase, a complex of the mitochondrial respiratory chain. The complex is composed of four nuclear-encoded subunits and is localized in the mitochondrial inner membrane. SDHA contains the FAD binding site where succinate is deprotonated and converted to fumarate. Mutations in this gene have been associated with a form of mitochondrial respiratory chain deficiency known as Leigh Syndrome. A pseudogene has been identified on chromosome 3q29. Alternatively spliced transcript variants encoding different isoforms have been found for this gene.[2]
Structure
The SDHA gene is located on the p arm of chromosome 5 at locus 15 and is composed of 16 exons.[2] The SDHA protein encoded by this gene is 664 amino acids long and weighs 72.7 kDA.[3][4]
SDHA protein has four subdomains, including capping domain, helical domain, C-terminal domain and most notably, β-barrel FAD-binding domain at N-terminus. Therefore, SDHA is a flavoprotein (Fp) due to the prosthetic group flavin adenine dinucleotide (FAD). Crystal structure suggests that FAD is covalently bound to a histidine residue (His99) and further coordinated by hydrogen bonds with number of other amino acid residues within the FAD-binding domain. FAD which is derived from riboflavin (vitamin B2) is thus essential cofactor for SDHA and whole complex II function.[5]
Function
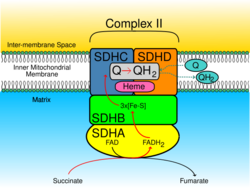
The SDH complex is located on the inner membrane of the mitochondria and participates in both the citric acid cycle and the respiratory chain. The succinate dehydrogenase (SDH) protein complex catalyzes the oxidation of succinate (succinate + ubiquinone => fumarate + ubiquinol). Electrons removed from succinate transfer to SDHA, transfer across SDHB through iron sulphur clusters to the SDHC/SDHD subunits on the hydrophobic end of the complex anchored in the mitochondrial membrane.
Initially, SDHA oxidizes succinate via deprotonation at the FAD binding site, forming FADH2 and leaving fumarate, loosely bound to the active site, free to exit the protein. The electrons derived from succinate tunnel along the [Fe-S] relay in the SDHB subunit until they reach the [3Fe-4S] iron sulfur cluster. The electrons are then transferred to an awaiting ubiquinone molecule at the Q pool active site in the SDHC/SDHD dimer. The O1 carbonyl oxygen of ubiquinone is oriented at the active site (image 4) by hydrogen bond interactions with Tyr83 of SDHD. The presence of electrons in the [3Fe-4S] iron sulphur cluster induces the movement of ubiquinone into a second orientation. This facilitates a second hydrogen bond interaction between the O4 carbonyl group of ubiquinone and Ser27 of SDHC. Following the first single electron reduction step, a semiquinone radical species is formed. The second electron arrives from the [3Fe-4S] cluster to provide full reduction of the ubiquinone to ubiquinol.[6]
SDHA acts as an intermediate in the basic SDH enzyme action:
- SDHA converts succinate to fumarate as part of the Citric Acid Cycle. This reaction also converts FAD to FADH2.
- Electrons from the FADH2 are transferred to the SDHB subunit iron clusters [2Fe-2S],[4Fe-4S],[3Fe-4S]. This function is part of the Respiratory chain
- Finally the electrons are transferred to the Ubiquinone (Q) pool via the SDHC/SDHD subunits.
Clinical significance
Bi-allelic mutations (i.e. both copies of the gene are mutated) have been described in Leigh syndrome, a progressive brain disorder that typically appears in infancy or early childhood. Affected children may experience vomiting, seizures, delayed development, muscle weakness, and problems with movement. Heart disease, kidney problems, and difficulty breathing can also occur in people with this disorder.[7] The SDHA gene mutations responsible for Leigh syndrome change single amino acids in the SDHA protein, such as a G555E mutation observed in multiple patients,[8][9] or result in an abnormally short protein. These genetic changes disrupt the activity of the SDH enzyme, impairing the ability of mitochondria to produce energy. It is not known, however, how mutations in the SDHA gene are related to the specific features of Leigh syndrome.
SDHA is a tumour suppressor gene, and heterozygous carriers have an increased risk of paragangliomas as well as pheochromocytomas and renal cancer.[10] Risk management for heterozygous carriers of an SDHA mutation can involve annual urine tests for metanephrines and 3-methoxytyramine and MRIs.[11]
Interactive pathway map
References
- ↑ "Human complex II (succinate-ubiquinone oxidoreductase): cDNA cloning of the flavoprotein (Fp) subunit of liver mitochondria". Journal of Biochemistry 116 (1): 221–7. July 1994. doi:10.1093/oxfordjournals.jbchem.a124497. PMID 7798181.
- ↑ 2.0 2.1 "Entrez Gene: succinate dehydrogenase complex". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=6389.
- ↑ "Integration of cardiac proteome biology and medicine by a specialized knowledgebase". Circulation Research 113 (9): 1043–53. October 2013. doi:10.1161/CIRCRESAHA.113.301151. PMID 23965338.
- ↑ "SDHA - Succinate dehydrogenase [ubiquinone flavoprotein subunit, mitochondrial"]. Cardiac Organellar Protein Atlas Knowledgebase (COPaKB). https://amino.heartproteome.org/web/protein/P31040.
- ↑ "Protein-mediated assembly of succinate dehydrogenase and its cofactors". Critical Reviews in Biochemistry and Molecular Biology 50 (2): 168–80. March–April 2015. doi:10.3109/10409238.2014.990556. PMID 25488574.
- ↑ "Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction". The Journal of Biological Chemistry 281 (11): 7309–16. March 2006. doi:10.1074/jbc.m508173200. PMID 16407191.
- ↑ "Leigh syndrome" (in en). U.S. National Library of Medicine. https://ghr.nlm.nih.gov/condition/leigh-syndrome.
- ↑ "Phenotypic variability of mitochondrial disease caused by a nuclear mutation in complex II". Molecular Genetics and Metabolism 89 (3): 214–21. November 2006. doi:10.1016/j.ymgme.2006.05.003. PMID 16798039.
- ↑ "Homozygous Gly555Glu mutation in the nuclear-encoded 70 kDa flavoprotein gene causes instability of the respiratory chain complex II". American Journal of Medical Genetics. Part A 120A (1): 13–8. July 2003. doi:10.1002/ajmg.a.10202. PMID 12794685.
- ↑ Reference, Genetics Home. "SDHA". https://ghr.nlm.nih.gov/gene/SDHA#conditions.
- ↑ Online, eviQ Cancer Treatments. "eviQ Cancer Treatments Online > eviQ home". https://www.eviq.org.au/.
Further reading
- "Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes". The Biochemical Journal 383 (Pt 2): 237–48. October 2004. doi:10.1042/BJ20040647. PMID 15242332.
- "Single nucleotide polymorphisms in succinate dehydrogenase subunits and citrate synthase genes: association results for impaired spermatogenesis". International Journal of Andrology 30 (3): 144–52. June 2007. doi:10.1111/j.1365-2605.2006.00730.x. PMID 17298551.
- "Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA)". Journal of Neurology, Neurosurgery, and Psychiatry 77 (1): 74–6. January 2006. doi:10.1136/jnnp.2005.067041. PMID 16361598.
- "Housekeeping genes for phylogenetic analysis of eutherian relationships". Molecular Biology and Evolution 23 (8): 1493–503. August 2006. doi:10.1093/molbev/msl027. PMID 16751257.
- "Regulation of succinate-ubiquinone reductase and fumarate reductase activities in human complex II by phosphorylation of its flavoprotein subunit". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences 85 (7): 258–65. 2009. doi:10.2183/pjab.85.258. PMID 19644226. Bibcode: 2009PJAB...85..258T.
- "SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma". Science 325 (5944): 1139–42. August 2009. doi:10.1126/science.1175689. PMID 19628817. Bibcode: 2009Sci...325.1139H.
- "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Research 16 (1): 55–65. January 2006. doi:10.1101/gr.4039406. PMID 16344560.
- "Frataxin interacts functionally with mitochondrial electron transport chain proteins". Human Molecular Genetics 14 (15): 2091–8. August 2005. doi:10.1093/hmg/ddi214. PMID 15961414.
- "Sequence variation in human succinate dehydrogenase genes: evidence for long-term balancing selection on SDHA". BMC Biology 5: 12. March 2007. doi:10.1186/1741-7007-5-12. PMID 17376234.
- "A role for mitochondrial enzymes in inherited neoplasia and beyond". Nature Reviews. Cancer 3 (3): 193–202. March 2003. doi:10.1038/nrc1013. PMID 12612654.
- "Succinate dehydrogenase deficiency in human". Cellular and Molecular Life Sciences 62 (19–20): 2317–24. October 2005. doi:10.1007/s00018-005-5237-6. PMID 16143825.
- "Patients with Leber hereditary optic neuropathy fail to compensate impaired oxidative phosphorylation". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1797 (2): 197–203. February 2010. doi:10.1016/j.bbabio.2009.10.003. PMID 19836344.
- "Genetic variants in nuclear-encoded mitochondrial genes influence AIDS progression". PLOS ONE 5 (9): e12862. September 2010. doi:10.1371/journal.pone.0012862. PMID 20877624. Bibcode: 2010PLoSO...512862H.
- "Homozygous Gly555Glu mutation in the nuclear-encoded 70 kDa flavoprotein gene causes instability of the respiratory chain complex II". American Journal of Medical Genetics. Part A 120A (1): 13–8. July 2003. doi:10.1002/ajmg.a.10202. PMID 12794685.
- "Polymorphisms in mitochondrial genes and prostate cancer risk". Cancer Epidemiology, Biomarkers & Prevention 17 (12): 3558–66. December 2008. doi:10.1158/1055-9965.EPI-08-0434. PMID 19064571.
- "Coupling mitochondrial respiratory chain to cell death: an essential role of mitochondrial complex I in the interferon-beta and retinoic acid-induced cancer cell death". Cell Death and Differentiation 14 (2): 327–37. February 2007. doi:10.1038/sj.cdd.4402004. PMID 16826196.
- "Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis". Molecular and Cellular Biochemistry 342 (1–2): 111–5. September 2010. doi:10.1007/s11010-010-0474-x. PMID 20440543.
- "Two compound frame-shift mutations in succinate dehydrogenase gene of a Chinese boy with encephalopathy". Brain & Development 36 (5): 394–8. May 2014. doi:10.1016/j.braindev.2013.06.003. PMID 23849264.
 |