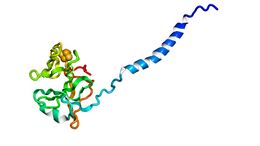
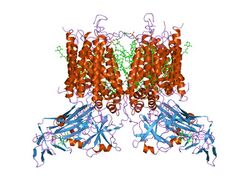
Biology:Rieske protein
Rieske proteins are iron–sulfur protein (ISP) components of cytochrome bc1 complexes and cytochrome b6f complexes and are responsible for electron transfer in some biological systems. John S. Rieske and co-workers first discovered the protein and in 1964 isolated an acetylated form of the bovine mitochondrial protein.[1] In 1979 Trumpower's lab isolated the "oxidation factor" from bovine mitochondria and showed it was a reconstitutively-active form of the Rieske iron-sulfur protein [2]
It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV.[3]
Biological function
Ubiquinol-cytochrome-c reductase (also known as bc1 complex or complex III) is an enzyme complex of bacterial and mitochondrial oxidative phosphorylation systems. It catalyses the oxidation-reduction reaction of the mobile components ubiquinol and cytochrome c, contributing to an electrochemical potential difference across the mitochondrial inner or bacterial membrane, which is linked to ATP synthesis.[4][5]
The complex consists of three subunits in most bacteria, and nine in mitochondria: both bacterial and mitochondrial complexes contain cytochrome b and cytochrome c1 subunits, and an iron–sulfur 'Rieske' subunit, which contains a high potential 2Fe-2S cluster.[6] The mitochondrial form also includes six other subunits that do not possess redox centres. Plastoquinone-plastocyanin reductase (b6f complex), present in cyanobacteria and the chloroplasts of plants, catalyses the oxidoreduction of plastoquinol and cytochrome f. This complex, which is functionally similar to ubiquinol-cytochrome c reductase, comprises cytochrome b6, cytochrome f and Rieske subunits.[7]
The Rieske subunit acts by binding either a ubiquinol or plastoquinol anion, transferring an electron to the 2Fe-2S cluster, then releasing the electron to the cytochrome c or cytochrome f heme iron.[4][7] The reduction of the Rieske center increases the affinity of the subunit by several orders of magnitude, stabilizing the semiquinone radical at the Q(P) site.[8] The Rieske domain has a [2Fe-2S] center. Two conserved cysteines coordinate one Fe ion while the other Fe ion is coordinated by two conserved histidines. The 2Fe-2S cluster is bound in the highly conserved C-terminal region of the Rieske subunit.
Rieske protein family
The homologues of the Rieske proteins include ISP components of cytochrome b6f complex, aromatic-ring-hydroxylating dioxygenases (phthalate dioxygenase, benzene, naphthalene and toluene 1,2-dioxygenases) and arsenite oxidase (EC 1.20.98.1). Comparison of amino acid sequences has revealed the following consensus sequence:
- Cys-Xaa-His-(Xaa)15–17-Cys-Xaa-Xaa-His
3D structure
The crystal structures of a number of Rieske proteins are known. The overall fold, comprising two subdomains, is dominated by antiparallel β-structure and contains variable numbers of α-helices. The smaller "cluster-binding" subdomains in mitochondrial and chloroplast proteins are virtually identical, whereas the large subdomains are substantially different in spite of a common folding topology. The [Fe2S2] cluster-binding subdomains have the topology of an incomplete antiparallel β-barrel. One iron atom of the Rieske [Fe2S2] cluster in the domain is coordinated by two cysteine residues and the other is coordinated by two histidine residues through the Nδ atoms. The ligands coordinating the cluster originate from two loops; each loop contributes one Cys and one His.
Subfamilies
- Rieske iron–sulfur protein, C-terminal InterPro: IPR005805
- Arsenite oxidase, small subunit InterPro: IPR014067
Human proteins containing this domain
AIFM3; RFESD; UQCRFS1;
References
- ↑ "Isolation and properties of an iron-protein from the (reduced coenzyme Q)-cytochrome C reductase complex of the respiratory chain". Biochem. Biophys. Res. Commun. 15 (4): 338–344. 1964. doi:10.1016/0006-291X(64)90171-8.
- ↑ "Purification of a reconstitutively active iron-sulfur protein (oxidation factor) from succinate:cytochrome c reductase complex of bovine heart mitochondria.". J Biol Chem 254 (17): 8697–706. 1979. doi:10.1016/S0021-9258(19)86947-8. PMID 224062.
- ↑ Brown, E.N. and Friemann, R. and Karlsson, A. and Parales, J.V. and Couture, M.M. and Eltis, L.D. and Ramaswamy, S. (2008). "Determining Rieske cluster reduction potentials". J. Biol. Inorg. Chem. 13 (8): 1301–1313. doi:10.1007/s00775-008-0413-4. PMID 18719951.
- ↑ Jump up to: 4.0 4.1 "The primary structure of the iron-sulfur subunit of ubiquinol-cytochrome c reductase from Neurospora, determined by cDNA and gene sequencing". Eur. J. Biochem. 149 (1): 95–9. May 1985. doi:10.1111/j.1432-1033.1985.tb08898.x. PMID 2986972. https://opus.bibliothek.uni-wuerzburg.de/frontdoor/index/index/docId/5806.
- ↑ "Nucleotide sequence and transcription of the fbc operon from Rhodopseudomonas sphaeroides. Evaluation of the deduced amino acid sequences of the FeS protein, cytochrome b and cytochrome c1". Eur. J. Biochem. 154 (3): 569–79. February 1986. doi:10.1111/j.1432-1033.1986.tb09437.x. PMID 3004982. https://nbn-resolving.org/urn:nbn:de:bvb:20-opus-62615.
- ↑ "The genes of the Paracoccus denitrificans bc1 complex. Nucleotide sequence and homologies between bacterial and mitochondrial subunits". J. Biol. Chem. 262 (28): 13805–11. October 1987. doi:10.1016/S0021-9258(19)76497-7. PMID 2820981.
- ↑ Jump up to: 7.0 7.1 "Import and processing of the precursor of the Rieske FeS protein of tobacco chloroplasts". Plant Mol. Biol. 20 (2): 289–99. October 1992. doi:10.1007/BF00014496. PMID 1391772.
- ↑ Link TA (July 1997). "The role of the 'Rieske' iron sulfur protein in the hydroquinone oxidation (Q(P)) site of the cytochrome bc1 complex. The 'proton-gated affinity change' mechanism". FEBS Lett. 412 (2): 257–64. doi:10.1016/S0014-5793(97)00772-2. PMID 9256231.
Further reading
- "Structure of a water soluble fragment of the 'Rieske' iron-sulfur protein of the bovine heart mitochondrial cytochrome bc1 complex determined by MAD phasing at 1.5 A resolution". Structure 4 (5): 567–79. May 1996. doi:10.1016/S0969-2126(96)00062-7. PMID 8736555.
- "Functional analysis in yeast of cDNA coding for the mitochondrial Rieske iron-sulfur protein of higher plants". Proc. Natl. Acad. Sci. U.S.A. 88 (23): 10716–20. December 1991. doi:10.1073/pnas.88.23.10716. PMID 1961737. Bibcode: 1991PNAS...8810716H.
- "The mitochondrial targeting presequence of the Rieske iron-sulfur protein is processed in a single step after insertion into the cytochrome bc1 complex in mammals and retained as a subunit in the complex". J. Biol. Chem. 268 (12): 8387–90. April 1993. doi:10.1016/S0021-9258(18)52883-0. PMID 8386158.
- Ferraro, D.J., Gakhar, L. and Ramaswamy, S. (2005). "Rieske business: structure-function of Rieske non-heme oxygenases". Biochem. Biophys. Res. Commun. 338 (1): 175–190. doi:10.1016/j.bbrc.2005.08.222. PMID 16168954.
- Mason, J.R.; Cammack, R. (1992). "The electron-transport proteins of hydroxylating bacterial dioxygenases". Annu. Rev. Microbiol. 46: 277–305. doi:10.1146/annurev.mi.46.100192.001425. PMID 1444257.
- Schmidt, C.L. (2004). "Rieske iron-sulfur proteins from extremophilic organisms". J. Bioenerg. Biomembr. 36 (1): 107–113. doi:10.1023/B:JOBB.0000019602.96578.78. PMID 15168614.
- Schneider, D.; Schmidt, C.L. (2005). "Multiple Rieske proteins in prokaryotes: where and why?". Biochim. Biophys. Acta 1710 (1): 1–12. doi:10.1016/j.bbabio.2005.09.003. PMID 16271700.
- Brown, E.N. and Friemann, R. and Karlsson, A. and Parales, J.V. and Couture, M.M. and Eltis, L.D. and Ramaswamy, S. (2008). "Determining Rieske cluster reduction potentials". J. Biol. Inorg. Chem. 13 (8): 1301–1313. doi:10.1007/s00775-008-0413-4. PMID 18719951.
External links
- PDB: 1RIE - X-ray structure of Rieske protein (water-soluble fragment) of the bovine mitochondrial cytochrome bc1 complex
- PDB: 1RFS - X-ray structure of Rieske protein (water-soluble fragment) of the spinach chloroplast cytochrome b6 fcomplex
- PDB: 1FQT - X-ray structure of Rieske-type ferredoxin associated with biphenyl dioxygenase from Burkholderia cepacia
- PDB: 1G8J - X-ray structure of Rieske subunit of arsenite oxidase from Alcaligenes faecalis
- PDB: 2I7F - X-ray structure of the Sphingomonas yanoikuyae B1 Rieske ferredoxin
- PDB: 2QPZ - X-ray structure of the Pseudomonas Naphthalene 1,2-dioxygenase Rieske ferredoxin
- InterPro: IPR005806 - InterPro entry for Rieske [2Fe-2S] region
 |