Biology:Sarcosine dehydrogenase
| Sarcosine dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.5.8.3 | ||||||||
| CAS number | 37228-65-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, sarcosine dehydrogenase (EC 1.5.8.3) is a mitochondrial enzyme that catalyzes the chemical reaction N-demethylation of sarcosine to give glycine.[1] This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH group of donor with other acceptors. The systematic name of this enzyme class is sarcosine:acceptor oxidoreductase (demethylating). Other names in common use include sarcosine N-demethylase, monomethylglycine dehydrogenase, and sarcosine:(acceptor) oxidoreductase (demethylating). Sarcosine dehydrogenase is closely related to dimethylglycine dehydrogenase, which catalyzes the demethylation reaction of dimethylglycine to sarcosine. Both sarcosine dehydrogenase and dimethylglycine dehydrogenase use FAD as a cofactor. Sarcosine dehydrogenase is linked by electron-transferring flavoprotein (ETF) to the respiratory redox chain.[2] The general chemical reaction catalyzed by sarcosine dehydrogenase is:
- sarcosine + acceptor + H2O [math]\displaystyle{ \rightleftharpoons }[/math] glycine + formaldehyde + reduced acceptor
Structure
There is no crystal structure available for sarcosine dehydrogenase. Sarcosine dehydrogenase contains a covalently bound FAD group " linked via the 8 alpha position of the isoalloxazine ring to an imidazole N(3) of a histidine residue".[3] The enzyme, according to Freisell Wr. et al., also contains non-heme iron in a ratio of 1 or 2 iron per 300000g of enzyme,[4] and 0.5 mol of acid soluble sulfur suggesting that the electron transfer during the first step in the reaction might proceed through a different pathway than that of Fe-S clusters.[3]
Mechanism
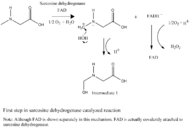
Sarcosine dehydrogenase, with sarcosine as its substrate, follows Michaelis–Menten kinetics and has a Km of 0.5 mM and a Vmax of 16 mmol/hr/mg protein.[8] The enzyme is inhibited competitively by methoxyacetic acid, which has a Ki of 0.26 mM [9]
The exact mechanism of sarcosine dehydrogenase is not available. However, according to the overall net reaction discussed in Honova.E, et al. paper:
- Sarcosine + H2O + O2 → glycine + formaldehyde + H2O2[10]
the first step of the reaction might involve the transfer of a hydride on the N-methyl group of sarcosine onto FAD, allowing H2O to attack the carbocation in order to form intermediate 1 (See figure 1). There is no deamination step. Instead, the demethylation of the N-methyl group on sarcosine occurs directly.[6] The reduced FADH− from the first step then is oxidized by O2 to form H2O2.[5]
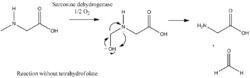
The demethylation of sarcosine catalyzed by sarcosine dehydrogenase can proceed with or without the presence of tetrahydrofolate.[11] Under anaerobic condition and without tetrahydrofolate, however, a free formaldehyde is formed after the N-demethylation of sarcosine.[12] The reaction with 1 mole of sarcosine and 1 mole of FAD, under this condition, yields 1 mole of glycine and 1 mole of formaldehyde (See figure 2 for mechanism).[9]
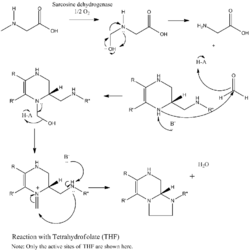
Under the presence of tetrahydrofolate, sarcosine dehydrogenase binds to tetrahydrofolate and convert tetrahydrofolate to 5,10-methylenetetrahydrofolate. Tetrahydrofolate here serves as a 1-carbon acceptor during the demethylation process (See figure 3 for mechanism).[2]
Function
Sarcosine dehydrogenase is one of the enzymes in sarcosine metabolism, which catalyzes the demethylation of sarcosine to make glycine. It is preceded by dimethylglycine dehydrogenase which turns dimethylglycine into sarcosine. Glycine can also be turned into sarcosine by glycine N-methyltransferase.[13] Since glycine is the production of sarcosine dehydrogenase catalyzed reaction, aside from sarcosine metabolism, the enzyme is also indirectly connected to the creatine cycle and the respiratory chain in the mitochondria [14][15][16] (See figure 4 for pathway). Even so, the biological significance of sarcosine dehydrogenase beyond sarcosine metabolism is not entirely known. In a study of hereditary hemochromatosis using both wild type and HFE (gene) deficient mice fed with 2 percent carbonyl iron supplemented diet, sarcosine dehydrogenase was shown to be down-regulated in HFE deficient mice, but role sarcosine dehydrogenase in iron metabolism is unknown from the experiment conducted.[17]
Disease relevance
Sarcosinemia
Sarcosinemia is an autosomal recessive disease caused by a mutation of the sarcosine dehydrogenase gene in the 9q33-q34 gene locus.[18] This leads to a compromised sarcosine metabolism and causes the build-up of sarcosine in blood and urine, a condition known as sarcosinemia.
Prostate cancer
In addition to sarcosinaemia, sarcosine dehydrogenase also seems to play a role in the progression process of prostate cancer. The concentration of sarcosine, along with those of uracil, kynurenine, glycerol 3-phosphate, leucine and proline increases as prostate cancer progresses. Thus, sarcosine can be used as a potential biomarker for the detection of prostate cancer and for measuring the progress of the disease.[19] As Sreekumar, A. et al.’s paper shows, the removal of sarcosine dehydrogenase from benign prostate epithelial cells increases the concentration of sarcosine and increase cancer cell invasions while the removal of either dimethylglycine dehydrogenase or glycine N-methyltransferase in prostate cancer cells decreases cell invasions. This demonstrates that sarcosine metabolism plays a key-role in prostate cancer cell invasion and migration. Sreekumar’s study suggests that sarcosine dehydrogenase and other enzymes in the sarcosine metabolism pathways could be potential therapeutic targets for prostate cancer.[13] However, a study done by Jentzmik F. et al. by analyzing sarcosine level in 92 patients with prostate cancer draws a different conclusion: sarcosine cannot be used as an indicator and biomarker for prostate cancer.[20]
See also
References
- ↑ "The effect of tetrahydrofolate on the reduction of electron transfer flavoprotein by sarcosine and dimethylglycine dehydrogenases". Biochem. J. 203 (3): 707–15. June 1982. doi:10.1042/bj2030707. PMID 6180732.
- ↑ Jump up to: 2.0 2.1 "Channelling and formation of 'active' formaldehyde in dimethylglycine oxidase". EMBO J. 22 (16): 4038–48. August 2003. doi:10.1093/emboj/cdg395. PMID 12912903.
- ↑ Jump up to: 3.0 3.1 "The amino acid sequences of the flavin-peptides of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver mitochondria". J. Biol. Chem. 260 (24): 12998–3002. October 1985. doi:10.1016/S0021-9258(17)38827-0. PMID 4055729.
- ↑ "Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase". J. Biol. Chem. 237: 94–8. January 1962. doi:10.1016/S0021-9258(18)81367-9. PMID 13895406.
- ↑ Jump up to: 5.0 5.1 "Catalysis of electron transfer during activation of O2 by the flavoprotein glucose oxidase". Proc. Natl. Acad. Sci. U.S.A. 100 (1): 62–7. January 2003. doi:10.1073/pnas.252644599. PMID 12506204.
- ↑ Jump up to: 6.0 6.1 "www.jbc.org". http://www.jbc.org/content/138/1/211.full.pdf.
- ↑ "Mechanisms of formylation and hydroxymethylation reactions". J Cell Comp Physiol 54: 109–25. December 1959. doi:10.1002/jcp.1030540410. PMID 14403792.
- ↑ "Identification of a covalently bound flavoprotein in rat liver mitochondria with sarcosine dehydrogenase". Biochem. Biophys. Res. Commun. 87 (3): 706–11. April 1979. doi:10.1016/0006-291X(79)92016-3. PMID 454421.
- ↑ Jump up to: 9.0 9.1 "Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver". Arch. Biochem. Biophys. 243 (2): 396–407. December 1985. doi:10.1016/0003-9861(85)90516-8. PMID 2417560.
- ↑ "Sarcosine dehydrogenase activity in liver mitochondria of infant and adult rats". Experientia 23 (8): 632–3. August 1967. doi:10.1007/bf02144166. PMID 6051684.
- ↑ "Identification of folate binding protein of mitochondria as dimethylglycine dehydrogenase". Proc. Natl. Acad. Sci. U.S.A. 77 (8): 4484–8. August 1980. doi:10.1073/pnas.77.8.4484. PMID 6159630.
- ↑ "Studies on the mechanism of the biosynthesis of serine". J. Biol. Chem. 196 (2): 599–614. May 1952. doi:10.1016/S0021-9258(19)52395-X. PMID 12981004.
- ↑ Jump up to: 13.0 13.1 "Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression". Nature 457 (7231): 910–4. February 2009. doi:10.1038/nature07762. PMID 19212411. PMC 2724746. https://deepblue.lib.umich.edu/bitstream/2027.42/62661/1/nature07762.pdf.
- ↑ Jump up to: 14.0 14.1 "Creatine and creatinine metabolism". Physiol. Rev. 80 (3): 1107–213. July 2000. doi:10.1152/physrev.2000.80.3.1107. PMID 10893433.
- ↑ Jump up to: 15.0 15.1 "Transport and metabolism of sarcosine in hypersarcosinemic and normal phenotypes". J. Clin. Invest. 50 (11): 2313–22. November 1971. doi:10.1172/JCI106729. PMID 5096515.
- ↑ Jump up to: 16.0 16.1 "Defect in dimethylglycine dehydrogenase, a new inborn error of metabolism: NMR spectroscopy study". Clin. Chem. 45 (4): 459–64. April 1999. doi:10.1093/clinchem/45.4.459. PMID 10102904.
- ↑ "Iron-independent specific protein expression pattern in the liver of HFE-deficient mice". Int. J. Biochem. Cell Biol. 39 (5): 1006–15. 2007. doi:10.1016/j.biocel.2007.01.021. PMID 17376729.
- ↑ "Cloning and mapping of the cDNA for human sarcosine dehydrogenase, a flavoenzyme defective in patients with sarcosinemia". Genomics 59 (3): 300–8. August 1999. doi:10.1006/geno.1999.5886. PMID 10444331.
- ↑ "Sarcosine as a potential prostate cancer biomarker and therapeutic target". Cancer Biol. Ther. 9 (5): 341–2. March 2010. doi:10.4161/cbt.9.5.11310. PMID 20150759.
- ↑ "Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression". J. Urol. 185 (2): 706–11. February 2011. doi:10.1016/j.juro.2010.09.077. PMID 21168877.
Further reading
- "Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase". J. Biol. Chem. 237: 94–8. 1962. doi:10.1016/S0021-9258(18)81367-9. PMID 13895406.
- "Solubilization and electron transfer flavoprtein requirement of mitochondrial sarcosine dehydrogenase and dimethylglycine dehydrogenase". J. Biol. Chem. 236: 177–83. 1961. doi:10.1016/S0021-9258(18)64450-3. PMID 13716069.
 |


