Biology:Methylenetetrahydrofolate reductase
 Generic protein structure example |
Methylenetetrahydrofolate reductase (MTHFR) is the rate-limiting enzyme in the methyl cycle, and it is encoded by the MTHFR gene.[1] Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine. Natural variation in this gene is common in otherwise healthy people. Although some variants have been reported to influence susceptibility to occlusive vascular disease, neural tube defects, Alzheimer's disease and other forms of dementia, colon cancer, and acute leukemia, findings from small early studies have not been reproduced. Some mutations in this gene are associated with methylenetetrahydrofolate reductase deficiency.[2][3][4] Complex I deficiency with recessive spastic paraparesis has also been linked to MTHFR variants. In addition, the aberrant promoter hypermethylation of this gene is associated with male infertility and recurrent spontaneous abortion.[5][6]
Biochemistry
| methylene tetrahydrofolate reductase [NAD(P)H] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Schematic diagram of the reductive carbon–nitrogen bond cleavage (represented by wavy line) catalyzed by methylenetetrahydrofolate reductase. | |||||||||
| Identifiers | |||||||||
| EC number | 1.5.1.20 | ||||||||
| CAS number | 9028-69-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
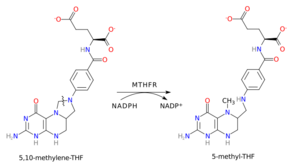
In the rate-limiting step of the methyl cycle, MTHFR irreversibly reduces 5,10-methylenetetrahydrofolate (substrate) to 5-methyltetrahydrofolate (product).
- 5,10-methylene tetrahydrofolate is used to convert dUMP to dTMP for de novo thymidine synthesis.
- 5-Methyltetrahydrofolate is used to convert homocysteine (a potentially toxic amino acid) to methionine by the enzyme methionine synthase. (Note that homocysteine can also be converted to methionine by the folate-independent enzyme betaine-homocysteine methyltransferase (BHMT).)
MTHFR contains a bound flavin cofactor and uses NAD(P)H as the reducing agent.
Structure
Mammalian MTHFR is composed of an N-terminal catalytic domain and a C-terminal regulatory domain. MTHFR has at least two promoters and two isoforms (70 kDa and 77 kDa).[7]
Regulation
MTHFR activity may be inhibited by binding of dihydrofolate (DHF)[8] and S-adenosylmethionine (SAM, or AdoMet).[9] MTHFR can also be phosphorylated – this decreases its activity by ~20% and allows it to be more easily inhibited by SAM.[10]
Genetics
The enzyme is coded by the gene with the symbol MTHFR on chromosome 1 location p36.3 in humans.[11] There are DNA sequence variants (genetic polymorphisms) associated with this gene. In 2000 a report brought the number of polymorphisms up to 24.[12] Two of the most investigated are C677T (rs1801133) and A1298C (rs1801131) single nucleotide polymorphisms (SNPs).
While multiple published studies have drawn relationships between these SNPs and a wide variety of diseases, the American College of Medical Genetics has issued an official Practice Guideline recommending against testing or reporting on these two variants, citing "Recent meta-analyses have disproven an association between hyperhomocysteinemia and risk for coronary heart disease and between MTHFR polymorphism status and risk for venous thromboembolism. There is growing evidence that MTHFR polymorphism testing has minimal clinical utility."[13]
C677T SNP (Ala222Val)
The MTHFR nucleotide at position 677 in the gene has two possibilities: C (cytosine) or T (thymine). C at position 677 (leading to an alanine at amino acid 222) is the reference allele. The 677T allele (leading to a valine substitution at amino acid 222) encodes a thermolabile alternative enzyme variant with reduced activity. Both reference and alternative genotypes are common, with the alternative allele frequency at 10-35%, depending on ancestry.[14]
Individuals with two copies of 677C (677CC) have the most common genotype. 677TT individuals (homozygous) have lower MTHFR activity than CC or CT (heterozygous) individuals. About ten percent of the North American population are T-homozygous for this polymorphism. There is ethnic variability in the frequency of the T allele – frequency in Mediterranean/Hispanics is greater than the frequency in Caucasians which, in turn, is greater than in Africans/African-Americans.[15]
The degree of enzyme thermolability (assessed as residual activity after heat inactivation) is much greater in 677TT individuals (18–22%) compared with 677CT (56%) and 677CC (66–67%).[16] Individuals of 677TT are predisposed to mild hyperhomocysteinemia (high blood homocysteine levels), because they have less active MTHFR available to produce 5-methyltetrahydrofolate (which is used to decrease homocysteine). Low dietary intake of the vitamin folate can also cause mild hyperhomocysteinemia.
Low folate intake affects individuals with the 677TT genotype to a greater extent than those with the 677CC/CT genotypes. 677TT (but not 677CC/CT) individuals with lower plasma folate levels are at risk for elevated plasma homocysteine levels.[17] In studies of human recombinant MTHFR, the protein encoded by 677T loses its FAD cofactor three times faster than the wild-type protein.[18] 5-Methyl-THF slows the rate of FAD release in both the wild-type and mutant enzymes, although it is to a much greater extent in the mutant enzyme.[18] Low folate status with the consequent loss of FAD enhances the thermolability of the enzyme, thus providing an explanation for the normalised homocysteine and DNA methylation levels in folate-replete 677TT individuals.
This polymorphism and mild hyperhomocysteinemia are associated with neural tube defects in offspring, increased risk for complications of pregnancy other complications of pregnancy,[19] arterial and venous thrombosis, and cardiovascular disease.[20] 677TT individuals are at a decreased risk for acute lymphoblastic leukemia[21] and colon cancer.[22]
Mutations in the MTHFR gene could be one of the factors leading to increased risk of developing schizophrenia.[23] Schizophrenic patients having the risk allele (T\T) show more deficiencies in executive function tasks.[24]
The C677T genotype used to be associated with increased risk of recurrent pregnancy loss (RPL) in non Caucasians,[25] however this link has been disproved in recent years.[citation needed] The American College of Medical Genetics recommendation guidelines currently state that people with recurrent pregnancy loss should not be tested for variants in the MTHFR gene.
There is also a tentative link between MTHFR mutations and dementia. One study of an elderly Japanese population[26] found correlations between the MTHFR 677CT mutation, an Apo E polymorphism, and certain types of senile dementia. Other research has found that individuals with folate-related mutations can still have a functional deficiency even when blood levels of folate are within the normal range,[27] and recommended supplementation of methyltetrahydrofolate to potentially prevent and treat dementia (along with depression). A 2011 study from China also found that the C677T SNP was associated with Alzheimer's disease in Asian populations (though not in Caucasians).[28]
C677T polymorphism is associated with risk of myocardial infarction in African, North American, and elderly populations.[29]
The CDC provides a web page with information on the "MTHFR Gene, Folic Acid, and Preventing Neural Tube Defects". National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention. 15 June 2022. https://www.cdc.gov/ncbddd/folicacid/mthfr-gene-and-folic-acid.html. Retrieved 24 Sep 2023.
A1298C SNP (Glu429Ala)
At nucleotide 1298 of the MTHFR, there are two possibilities: A or C. 1298A (leading to a Glu at amino acid 429) is the most common while 1298C (leading to an Ala substitution at amino acid 429) is less common. 1298AA is the "normal" homozygous, 1298AC the heterozygous, and 1298CC the homozygous for the "variant". In studies of human recombinant MTHFR, the protein encoded by 1298C cannot be distinguished from 1298A in terms of activity, thermolability, FAD release, or the protective effect of 5-methyl-THF.[18] The C mutation does not appear to affect the MTHFR protein. It does not result in thermolabile MTHFR and does not appear to affect homocysteine levels. It does, however, affect the conversion of MTHF to BH4 (tetrahydrobiopterin), an important cofactor in the production of neurotransmitters, and the synthesis of nitric oxide.[citation needed]
There has been some commentary on a 'reverse reaction' in which tetrahydrobiopterin (BH4) is produced when 5-methyltetrahydrofolate is converted back into methylenetetrahydrofolate. This however is not universally agreed upon. That reaction is thought to require 5-MTHF and SAMe.[citation needed] An alternative opinion is that 5-MTHF processes peroxynitrite, thereby preserving existing BH4, and that no such 'reverse reaction' occurs.
A maternal MTHFR A1298C polymorphism is associated with Down syndrome pregnancy. Subgroup and sensitivity analysis results showed that this polymorphism is a risk factor for Down syndrome pregnancy in Asian populations but not in Caucasian population as well as in overall meta-analysis.[30]
MTHFR A1298C may play a role as either a driver in the development of major depressive disorder or as a predictive or diagnostic marker, possibly in combination with C677T.[31]
Detection of MTHFR polymorphisms
A triplex tetra-primer ARMS-PCR method was developed for the simultaneous detection of C677T and A1298C polymorphisms with the A66G MTRR polymorphism in a single PCR reaction.[32]
Severe MTHFR deficiency
Severe MTHFR deficiency is rare (about 50 cases worldwide) and caused by mutations resulting in 0–20% residual enzyme activity.[12] Patients exhibit developmental delay, motor and gait dysfunction, seizures, and neurological impairment and have extremely high levels of homocysteine in their plasma and urine as well as low to normal plasma methionine levels. This deficiency and mutations in MTHFR have also been linked to recessive spastic paraparesis with complex I deficiency.[33]
A study on the Chinese Uyghur population indicated that rs1801131 polymorphism in MTHFR was associated with nsCL/P in Chinese Uyghur population. Given the unique genetic and environmental characters of the Uyghur population, these findings may be helpful for exploring the pathogenesis of this complex disease.[34]
Epigenetics
The MTHFR aberrant promoter hypermethylation is associated with male infertility. Furthermore, this improper epigenetic phenomenon was observed in semen samples of infertile males belonging to couples with a history of recurrent spontaneous abortion.[5] The MTHFR improper promoter hypermethylation may affect the two essential roles of DNA methylation in spermatogenetic cells, the global genome methylation process and the genomic imprinting of paternal genes. In addition, MTHFR gene promoter hypermethylation has also been associated with methylation loss at H19 imprinted gene in semen samples from infertile males.[6]
As a drug target
Inhibitors of MTHFR and antisense knockdown of the expression of the enzyme have been proposed as treatments for cancer.[35] The active form of folate, L-methylfolate, may be appropriate to target for conditions affected by MTHFR polymorphisms.[36]
Reaction and metabolism
The overall reaction catalyzed by MTHFR is illustrated on the right. The reaction uses an NAD(P)H hydride donor and an FAD cofactor. The E. coli enzyme has a strong preference for the NADH donor, whereas the mammalian enzyme is specific to NADPH.

Alternative medicine
With the growth of direct-to-consumer genetic testing, the alternative medicine industry has aggressively targeted a range of dubious tests[37] and highly profitable quack treatments for claimed MTHFR polymorphisms, despite the lack of any demonstrated health effects of these mutations.[38] The promotion of supplements and other treatments for MTHFR polymorphisms, especially centered on autistic spectrum disorder,[39] have been characterised as snake oil. Tests for MTHFR, while gaining popularity, are generally unnecessary because the association of MTHFR gene mutations with various diseases have not been established as clear-cut cause-and-effect relationship.[40]
See also
References
- ↑ "Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification". Nature Genetics 7 (2): 195–200. June 1994. doi:10.1038/ng0694-195. PMID 7920641.
- ↑ "Entrez Gene: MTHFR methylene tetrahydrofolate reductase (NAD(P)H)". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4524.
- ↑ "Molecular biology of 5,10-methylenetetrahydrofolate reductase". Journal of Nephrology 13 (1): 20–33. 2000. PMID 10720211.
- ↑ "Methylenetetrahydrofolate reductase: biochemical characterization and medical significance". Current Pharmaceutical Design 19 (14): 2574–93. 2013. doi:10.2174/1381612811319140008. PMID 23116396.
- ↑ 5.0 5.1 "Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion". Human Reproduction 27 (12): 3632–8. December 2012. doi:10.1093/humrep/des319. PMID 23010533.
- ↑ 6.0 6.1 "Methylation loss at H19 imprinted gene correlates with methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples from infertile males". Epigenetics 8 (9): 990–7. September 2013. doi:10.4161/epi.25798. PMID 23975186.
- ↑ "Multiple transcription start sites and alternative splicing in the methylenetetrahydrofolate reductase gene result in two enzyme isoforms". Mammalian Genome 13 (9): 483–92. September 2002. doi:10.1007/s00335-002-2167-6. PMID 12370778.
- ↑ "Modulation of methylenetetrahydrofolate reductase activity by S-adenosylmethionine and by dihydrofolate and its polyglutamate analogues". Advances in Enzyme Regulation 20: 123–31. 1982. doi:10.1016/0065-2571(82)90012-7. PMID 7051769. https://deepblue.lib.umich.edu/bitstream/2027.42/24098/1/0000355.pdf.
- ↑ "Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium". The Journal of Biological Chemistry 262 (6): 2485–93. February 1987. doi:10.1016/S0021-9258(18)61530-3. PMID 3818603. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=3818603.
- ↑ "Regulation of human methylenetetrahydrofolate reductase by phosphorylation". Proceedings of the National Academy of Sciences of the United States of America 102 (30): 10454–9. July 2005. doi:10.1073/pnas.0504786102. PMID 16024724. Bibcode: 2005PNAS..10210454Y.
- ↑ "Human methylenetetrahydrofolate reductase: isolation of cDNA mapping and mutation identification". Nature Genetics 7 (4): 551. August 1994. doi:10.1038/ng0894-551a. PMID 7951330.
- ↑ 12.0 12.1 "Characterization of six novel mutations in the methylenetetrahydrofolate reductase (MTHFR) gene in patients with homocystinuria". Human Mutation 15 (3): 280–7. 2000. doi:10.1002/(SICI)1098-1004(200003)15:3<280::AID-HUMU9>3.0.CO;2-I. PMID 10679944.
- ↑ "ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing". Genetics in Medicine 15 (2): 153–156. February 2013. doi:10.1038/gim.2012.165. PMID 23288205.
- ↑ "rs1801133". National Library of Medicine. https://www.ncbi.nlm.nih.gov/snp/rs1801133.
- ↑ "Worldwide distribution of a common methylenetetrahydrofolate reductase mutation". American Journal of Human Genetics 62 (5): 1258–60. May 1998. doi:10.1086/301836. PMID 9545406.
- ↑ "A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase". Nature Genetics 10 (1): 111–3. May 1995. doi:10.1038/ng0595-111. PMID 7647779. http://digitalcommons.unl.edu/lawfacpub/124.
- ↑ "MTHFR 677TT genotype and disease risk: is there a modulating role for B-vitamins?". The Proceedings of the Nutrition Society 73 (1): 47–56. February 2014. doi:10.1017/S0029665113003613. PMID 24131523.
- ↑ 18.0 18.1 18.2 "Effects of common polymorphisms on the properties of recombinant human methylenetetrahydrofolate reductase". Proceedings of the National Academy of Sciences of the United States of America 98 (26): 14853–8. December 2001. doi:10.1073/pnas.261469998. PMID 11742092. Bibcode: 2001PNAS...9814853Y.
- ↑ "The C677T methylenetetrahydrofolate reductase variant and third trimester obstetrical complications in women with unexplained elevations of maternal serum alpha-fetoprotein". Reproductive Biology and Endocrinology 2 (1): 65. September 2004. doi:10.1186/1477-7827-2-65. PMID 15352998.
- ↑ "Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences". American Journal of Pharmacogenomics 1 (3): 189–201. 2001. doi:10.2165/00129785-200101030-00004. PMID 12083967.
- ↑ "Methylenetetrahydrofolate reductase C677T and overall survival in pediatric acute lymphoblastic leukemia: a systematic review". Leukemia & Lymphoma 55 (1): 67–73. January 2014. doi:10.3109/10428194.2013.792336. PMID 23550988.
- ↑ "Folate, methyl-related nutrients, alcohol, and the MTHFR 677C-->T polymorphism affect cancer risk: intake recommendations". The Journal of Nutrition 133 (11 Suppl 1): 3748S–3753S. November 2003. doi:10.1093/jn/133.11.3748S. PMID 14608109.
- ↑ "Meta-Analysis of All Published Schizophrenia-Association Studies (Case-Control Only) for rs1801133 (C677T) polymorphism, MTHFR gene". Schizophrenia Research Forum. http://www.schizophreniaforum.org/res/sczgene/meta.asp?geneID=4.
- ↑ "Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia". Schizophrenia Research 92 (1–3): 181–8. May 2007. doi:10.1016/j.schres.2007.01.003. PMID 17344026.
- ↑ "Association between the MTHFR C677T polymorphism and recurrent pregnancy loss: a meta-analysis". Genetic Testing and Molecular Biomarkers 16 (7): 806–11. July 2012. doi:10.1089/gtmb.2011.0318. PMID 22313097.
- ↑ "Apolipoprotein E, methylenetetrahydrofolate reductase (MTHFR) mutation and the risk of senile dementia--an epidemiological study using the polymerase chain reaction (PCR) method". Journal of Epidemiology 10 (3): 163–72. May 2000. doi:10.2188/jea.10.163. PMID 10860300.
- ↑ "The role of folate in depression and dementia". The Journal of Clinical Psychiatry 68 (Suppl 10): 28–33. 2007. PMID 17900207. http://article.psychiatrist.com/?ContentType=START&ID=10003230.
- ↑ "Association between the MTHFR gene and Alzheimer's disease: a meta-analysis". The International Journal of Neuroscience 121 (8): 462–71. August 2011. doi:10.3109/00207454.2011.578778. PMID 21663380.
- ↑ "C667T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene and susceptibility to myocardial infarction: A systematic review and meta-analysis". International Journal of Cardiology 217: 99–108. August 2016. doi:10.1016/j.ijcard.2016.04.181. PMID 27179899.
- ↑ "Null association of maternal MTHFR A1298C polymorphism with Down syndrome pregnancy: An updated meta-analysis". Egyptian Journal of Medical Human Genetics 18 (1): 9–18. January 2017. doi:10.1016/j.ejmhg.2016.04.003.
- ↑ "Methylenetetrahydrofolate Reductase A1298C Polymorphism and Major Depressive Disorder". Cureus 9 (10): e1734. October 2017. doi:10.7759/cureus.1734. PMID 29209581.
- ↑ "Triplex tetra-primer ARMS-PCR method for the simultaneous detection of MTHFR c.677C>T and c.1298A>C, and MTRR c.66A>G polymorphisms of the folate-homocysteine metabolic pathway". Molecular and Cellular Probes 26 (1): 16–20. February 2012. doi:10.1016/j.mcp.2011.10.005. PMID 22074746.
- ↑ "Recessive spastic paraparesis associated with complex I deficiency due to MTHFR mutations". Journal of Neurology, Neurosurgery, and Psychiatry 83 (1): 115. January 2012. doi:10.1136/jnnp.2010.218586. PMID 21131308.
- ↑ "Association of MTHFR polymorphisms with nsCL/P in Chinese Uyghur population.". Egyptian Journal of Medical Human Genetics 17 (4): 311–316. 2016. doi:10.1016/j.ejmhg.2016.03.003.
- ↑ "Methylenetetrahydrofolate reductase (MTHFR): a novel target for cancer therapy". Current Pharmaceutical Design 14 (11): 1143–50. 2008. doi:10.2174/138161208784246171. PMID 18473861.
- ↑ "Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial". The Journal of Clinical Psychiatry 75 (8): 855–63. August 2014. doi:10.4088/JCP.13m08947. PMID 24813065.
- ↑ "Dubious MTHFR genetic mutation testing". 2015-06-11. https://sciencebasedmedicine.org/dubious-mthfr-genetic-mutation-testing/.
- ↑ "How Your Genetic Sequence Can Be Exploited By The Supplement Industry". 2016-11-14. https://www.forbes.com/sites/brittmariehermes/2016/11/14/genetic-sequence-exploited-supplement-industry/.
- ↑ "Autism cures promised by DNA testers belied by regulators". 2012-12-24. https://www.independent.co.uk/news/world/americas/autism-cures-promised-by-dna-testers-belied-by-regulators-8430767.html.
- ↑ "A Genetic Test You Don't Need". 2013-09-27. https://health.clevelandclinic.org/a-genetic-test-you-dont-need/.
Further reading
- "ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing". Genetics in Medicine 15 (2): 153–6. February 2013. doi:10.1038/gim.2012.165. PMID 23288205.
- "Methylenetetrahydrofolate reductase: a common human polymorphism and its biochemical implications". Chemical Record 2 (1): 4–12. 2003. doi:10.1002/tcr.10006. PMID 11933257. https://deepblue.lib.umich.edu/bitstream/2027.42/35288/1/10006_ftp.pdf.
- "Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences". American Journal of Pharmacogenomics 1 (3): 189–201. 2002. doi:10.2165/00129785-200101030-00004. PMID 12083967.
- "Methylene tetrahydrofolate reductase gene and coronary artery disease". The Journal of the Pakistan Medical Association 53 (1): 33–6. January 2003. PMID 12666851.
- "Folate, methyl-related nutrients, alcohol, and the MTHFR 677C-->T polymorphism affect cancer risk: intake recommendations". The Journal of Nutrition 133 (11 Suppl 1): 3748S–3753S. November 2003. doi:10.1093/jn/133.11.3748S. PMID 14608109.
- "Roles of methylenetetrahydrofolate reductase C677T polymorphism in repeated pregnancy loss". Clinical and Applied Thrombosis/Hemostasis 11 (3): 343–5. July 2005. doi:10.1177/107602960501100315. PMID 16015422.
- "Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis". Molecular Psychiatry 11 (2): 143–9. February 2006. doi:10.1038/sj.mp.4001746. PMID 16172608.
- "The thermolabile variant of MTHFR is associated with depression in the British Women's Heart and Health Study and a meta-analysis". Molecular Psychiatry 11 (4): 352–60. April 2006. doi:10.1038/sj.mp.4001790. PMID 16402130.
- "5,10-Methylenetetrahydrofolate reductase polymorphisms and acute lymphoblastic leukemia risk: a meta-analysis". Cancer Epidemiology, Biomarkers & Prevention 15 (10): 1956–63. October 2006. doi:10.1158/1055-9965.EPI-06-0334. PMID 17035405.
- "[Molecular genetics of MTHFR: polymorphisms are not all benign"]. Médecine/Sciences 23 (3): 297–302. March 2007. doi:10.1051/medsci/2007233297. PMID 17349292. http://www.medecinesciences.org/articles/medsci/pdf/2007/04/medsci2007233p297.pdf.
External links
- Overview of all the structural information available in the PDB for UniProt: P42898 (Methylenetetrahydrofolate reductase) at the PDBe-KB.
 |

