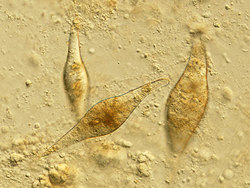
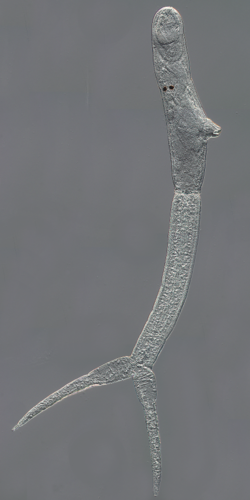
Biology:Trichobilharzia regenti
| Trichobilharzia regenti | |
|---|---|
| |
| Trichobilharzia regenti, cercaria | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Trematoda |
| Order: | Diplostomida |
| Family: | Schistosomatidae |
| Genus: | Trichobilharzia |
| Species: | T. regenti
|
| Binomial name | |
| Trichobilharzia regenti Horák, Kolářová & Dvořák, 1998 [1]
| |
Trichobilharzia regenti is a neuropathogenic parasitic flatworm of birds which also causes cercarial dermatitis in humans.[2] The species was originally described in 1998 in the Czech Republic[1] and afterwards it was detected also in other European countries, e.g. Denmark ,[3] Germany ,[4] France ,[5] Iceland,[6] Poland ,[7][8] Switzerland ,[9] or Russia ,[10] and even in Iran.[11][12] For its unique neurotropic behaviour in vertebrate hosts, the host-parasite interactions are extensively studied in terms of molecular biology, biochemistry and immunology.[13][14][15]
Life cycle
The life cycle of T. regenti is analogous to that of human schistosomes. Adult flukes mate in a nasal mucosa of anatid birds (e.g. Anas platyrhynchos, Spatula clypeata or Cairina moschata) and produce eggs with miracidia which hatch directly in the host tissue and leak outside when the bird is drinking/feeding.[1] Once in water, the miracidia swim using their cilia and actively search for a proper molluscan intermediate host (Radix lagotis, Radix labiata, Radix peregra).[16] In the snail, the miracidia develop into a primary sporocyst in which secondary sporocysts are formed and give rise to cercariae later on.[17]
Cercariae, infective larvae, exit the snail and penetrate the skin of an avian host. After penetration of the host's skin, they shed the immunogenic surface glycocalyx[18] and transform to schistosomula (subadult stage, sg. schistosomulum). Schistosomula then look for peripheral nerves to use them to get to the spinal cord. Through it they continue their migration to the brain[19][20] and, finally, the nasal tissue in a bill. Here, they mature, copulate and lay eggs while causing pathology (inflammatory infiltration, haemorrhages).[21]
If mammals are infected by cercariae (instead of birds), the parasites die in the skin being entrapped by immune response.[22] The clinical manifestation of such infection is known as a neglected allergic disease called cercarial dermatitis (or swimmer's itch).[2][23] In mice, especially in immunodeficient ones, migration of the parasite to the spinal cord was observed.[24][25]
A complete life cycle of T. regenti can be maintained under laboratory conditions using Radix lagotis and the Domestic duck (Anas platyrhynchos f. domestica) as intermediate and definitive hosts, respectively.[1] Interestingly, domestic ducks can also serve as reservoir hosts in aquaculture sites, such as rice fields.[12] To study biology of T. regenti in mammals, C57BL/6, BALB/c a SCID mouse strains are used as accidental hosts.[25][26][27]
Migration in vertebrate hosts
When cercariae of T. regenti find either avian or mammalian host, they penetrate its skin. For this purpose, they are equipped with cysteine peptidases present in their excretory/secretory products, which are capable of keratin and collagen degradation.[28][29] Experiments with laboratory prepared recombinant form of the cysteine peptidase cathepsin B2 of T. regenti (TrCB2) confirmed its ability to cleave skin proteins (collagen, keratin and elastin).[30]
After penetration the skin, cercariae transform to schistosomula and start a migration through the host's body. They avoid penetration into blood capillaries and rather prefer entering peripheral nerves in host's limbs. Schistosomula are found in peripheral nerves of ducks and mice as soon as 1.5 and 1 day post infection (DPI), respectively.[20] In both types of hosts, schistosomula exhibit a high affinity to the central nervous system which they enter via spinal roots.[26] Based on recent observation by 3D imaging techniques (ultramicroscopy and micro-CT), schistosomula appear to migrate preferably through the white matter of the spinal cord in both birds and mammals.[27]
The next course of the infection differs in final and accidental hosts. In ducks, schistosomula are observed in synsacral segments of a spinal cord 3 DPI and 7–8 days later (10–11 DPI) they reach the brain. In their final localisation (the nasal tissue), they occur 13–14 DPI and laying eggs starts 15 DPI.[20][21] In mice, the first schistosomula are found in a lumbar spinal cord as early as 2 DPI and medulla oblongata is invaded the day after, but only in some individuals. Most of schistosomula stay localised in the thoracic and cervical spinal cord and only exceptionally migrate to the brain.[19][20] Neither the presence of worms has been detected in a nasal cavity nor has their maturation been noticed in the nervous tissue. Schistosomula development in mice is suppressed likely due to the host immune response and/or the presence/absence of some essential (nutritional, stimulatory) host factors.[25]
Pathology in vertebrate hosts
In vertebrate hosts infected by T. regenti, pathological states might be caused by:
- penetrating cercariae transforming to schistosomula in the skin,
- schistosomula migrating through the central nervous system (CNS),
- adults laying eggs in the nasal mucosa (only in avian hosts).
Although mice are accidental hosts, most of the studies dealing with the pathological effects of T. regenti were conducted on this model.
Skin pathology
In the initial phase of the infection, early transformed schistosomula are localised in the skin. Information about pathology in the skin of birds has not been completed yet. In mice, immediate oedema and thickening of the site appear as early as 30 minutes after the penetration of cercariae; erythema is evident as well. Within 48 hours, inflammatory foci containing neutrophils, eosinophils, macrophages, CD4+ lymphocytes and degranulating mast cells develop around the parasites.[22][24]
In case of repeated infections, the cellular infiltration is substantially elevated and the extensive inflammation may lead to formation of large abscesses or even epidermal and/or dermal necrosis.[22] In humans, the clinical symptoms of cercarial penetration consist of macules/papules formation at the sites where the parasite entered the skin accompanied by intensive itching. The manifestation is more severe in previously sensitised people. This disease, caused not only by T. regenti but also by cercariae of other bird schistosome species, is called cercarial dermatitis (aka swimmer's itch). It is regarded as a neglected allergic disease.[2][23]
CNS pathology
The next phase of T. regenti infection is represented by schistosomula migration in the central nervous system.[15] This is accompanied by serious neurological malfunctions in birds that suffer from leg paralysis and balance disorders.[19] In ducks, eosinophilic meningitis with parasites surrounded by eosinophils and heterophils was noted. Additionally, leukocytes infiltrated perivascular spaces and tissues adjacent to the central canal.[31]
At this stage, schistosomula feed on nervous tissue as demonstrated by detection of oligodendrocytes and neurons in the lumen of parasite's intestine.[26] A cysteine peptidase cathepsin B1 of T. regenti (TrCB1) localised in intestines of migrating schistosomula is capable of myelin basic protein degradation, thus probably serving for nervous tissue digestion.[32] Nonetheless, the nervous tissue ingestion has likely only a minor pathogenic effect on the host central nervous tissue.[26] This is underpinned by observations of leg paralysis only in immunocompromised hosts,[24][26] whereas in experiments with immunocompetent mouse strains, the infected animals did not reveal any neurological disorders.[20][24][26] The neurological symptoms originate probably in mechanical damage of the nervous tissue leading to dystrophic or even necrotic changes of neurons and axonal injury. The cause of it is large migrating schistosomula (approximately 340×80 μm) which are not destroyed by proper immune response.[24][26][31] Recently, massive downregulation of neurophysiological pathways was suggested to be responsible for the motor dysfunction detected also in properly examined immunocompetent mice.[15]
Nasal pathology
In avian hosts, T. regenti reaches the nasal tissue where it mates and lay eggs. The gross pathology at this site consists of focal haemorrhages dispersed all over the mucosa. Infiltrates of lymphocytes are present around the eggs and even granulomas containing lymphocytes, eosinophils and heterophils form at later phases. Similar infiltrates are present around free miracidia, but the granuloma formation was not recorded. No cell reaction was noted in the vicinity of adult worms.[21]
Immune response in vertebrate hosts
Ducks
The records on cellular immune response to T. regenti in ducks are rather scarce. Cell infiltration of affected skin sites in repeatedly infected ducks was only noted, however, lacking further characterization of the infiltrating cells.[33] In the CNS, eosinophils and heterophils surround the parasite but do not prevent its migration towards the final localisation in the nasal mucosa.[19]
Considering antibody response, anti-cercarial IgM culminates 15 DPI, while anti-cercarial IgY reaches a peak 30 DPI. Ducks infected at higher age have higher anti-cercarial IgY levels than those infected at lower age. However, the anti-cercarial IgY levels are not largely dependent on the infection dose. Several parasite antigens recognized specifically by host IgY are considered as candidates for immunodiagnostics.[34]
Mice
The infection manifests as early type I hypersensitivity reaction and a late phase cutaneous inflammation.
Cellular immune response is represented by production of pro-inflammatory (IL-1β, IL-6 and IL-12p40) and anti-inflammatory (IL-10) cytokines in a skin of mice infected for the first time.[22] Lymphocytes from their skin draining lymph nodes exhibit mixed Th1/Th2 polarization after exposure to parasite antigens.[22][35] On the contrary, anti-inflammatory IL-4 and IL-10 dominate in mice infected repeatedly which also secrete large amounts of histamine from mast cells. Lymphocytes from their skin draining lymph nodes produce IL-4 and IL-5 after stimulation with parasite antigens which shows Th2 polarization of host immune response.[22]
In a spinal cord, strong cellular immune response consisting of granulocytes, plasma cells, macrophages, and T-cells develops in immunocompetent mice especially around the damaged schistosomula. CD3-deficient mice develop no or just mild inflammation which is accompanied by neurological symptoms due to mechanical damage caused to the nervous tissue.[24][26][31] Activated microglia are localised in the migratory tracks of schistosomula and in the inflammatory lesions containing parasite residues. Although microglia were suggested to take part in schistosomula destruction, they have anti-inflammatory M2 phenotype and rather facilitate the tissue repair.[15] Hypertrophied astrocytes are located in the migratory tracks and in the proximity of the schistosomula which implies their role in immune response and tissue reparation.[26] Murine astrocytes and microglia were shown to produce pro-inflammatory cytokines (IL-6 and TNF-α) and nitric oxide after in vitro exposure to parasite antigens, which supports their role in host immune response.[36]
IgM antibody response targets mainly carbohydrate epitopes of parasite molecules.[37] Parasite-specific IgG1 and IgG2a are present as soon as 7 DPI.[35] High levels of IgG1 and IgG2b, but no IgG2a, specific to mostly protein epitopes of cercarial homogenate are detectable as long as 150 DPI in repeatedly infected mice. The level of total IgE increases as soon as 10 DPI and remains high up to 150 DPI in reinfected mice.[37] Increased production of antigen-specific IgG1 and total IgE, but slight decrease in antigen-specific IgG2b corroborate Th2 immune polarization in repeatedly infected individuals.[22][37]
Humans
Clinical manifestation of human immune response to T. regenti infection is known as cercarial dermatitis (aka swimmer's itch).[2][23] Majority of humans (82% of adults, 57% of children) who have experienced cercarial dermatitis (caused by undetermined species of bird schistosome) have increased levels of T. regenti antigen-specific IgG, but not IgE. Cercarial homogenate and excretory-secretory products of T. regenti induce basophils from humans without a history of cercarial dermatitis to degranulate and release IL-4.[37]
References
- ↑ 1.0 1.1 1.2 1.3 "Trichobilharzia regenti n. sp. (Schistosomatidae, Bilharziellinae), a new nasal schistosome from Europe". Parasite 5 (4): 349–57. December 1998. doi:10.1051/parasite/1998054349. PMID 9879557.

- ↑ 2.0 2.1 2.2 2.3 "Avian schistosomes and outbreaks of cercarial dermatitis". Clinical Microbiology Reviews 28 (1): 165–90. January 2015. doi:10.1128/CMR.00043-14. PMID 25567226.
- ↑ "Molecular diversity of avian schistosomes in Danish freshwater snails". Parasitology Research 115 (3): 1027–37. March 2016. doi:10.1007/s00436-015-4830-3. PMID 26573519.
- ↑ "Having bird schistosomes in mind-the first detection of Bilharziella polonica (Kowalewski 1895) in the bird neural system". Parasitology Research 116 (3): 865–870. March 2017. doi:10.1007/s00436-016-5359-9. PMID 28012027.
- ↑ "Final hosts and variability of Trichobilharzia regenti under natural conditions". Parasitology Research 107 (4): 923–30. September 2010. doi:10.1007/s00436-010-1953-4. PMID 20556426.
- ↑ "Morphological features of the nasal blood fluke Trichobilharzia regenti (Schistosomatidae, Digenea) from naturally infected hosts". Parasitology Research 110 (5): 1881–92. May 2012. doi:10.1007/s00436-011-2713-9. PMID 22146993.
- ↑ "Bird schistosomes of wildfowl in the Czech Republic and Poland". Folia Parasitologica 54 (2): 88–93. June 2007. doi:10.14411/fp.2007.011. PMID 17886736.
- ↑ "Agents of swimmer's itch-dangerous minority in the Digenea invasion of Lymnaeidae in water bodies and the first report of Trichobilharzia regenti in Poland". Parasitology Research 117 (12): 3695–3704. December 2018. doi:10.1007/s00436-018-6068-3. PMID 30215139.
- ↑ "Genetic variability among cercariae of the Schistosomatidae (Trematoda: Digenea) causing swimmer's itch in Europe". Parasite 8 (3): 237–242. September 2001. doi:10.1051/parasite/2001083237. PMID 11584754.
- ↑ "Detection of European Trichobilharzia schistosomes ( T. franki, T. szidati, and T. regenti ) based on novel genome sequences". The Journal of Parasitology 96 (4): 802–6. August 2010. doi:10.1645/GE-2297.1. PMID 20677938.
- ↑ "Phylogenetic analysis of nasal avian schistosomes (Trichobilharzia) from aquatic birds in Mazandaran Province, northern Iran". Parasitology International 65 (2): 151–8. April 2016. doi:10.1016/j.parint.2015.11.009. PMID 26631753.
- ↑ 12.0 12.1 "Genetic diversity of an avian nasal schistosome causing cercarial dermatitis in the Black Sea-Mediterranean migratory route". Parasitology Research 117 (12): 3821–3833. December 2018. doi:10.1007/s00436-018-6087-0. PMID 30343420.
- ↑ "Laboratory of Helminthology". Czech Republic: Charles University in Prague. http://www.helminthology.cz/.
- ↑ "Comparative Transcriptomic Exploration Reveals Unique Molecular Adaptations of Neuropathogenic Trichobilharzia to Invade and Parasitize Its Avian Definitive Host". PLOS Neglected Tropical Diseases 10 (2): e0004406. February 2016. doi:10.1371/journal.pntd.0004406. PMID 26863542.
- ↑ 15.0 15.1 15.2 15.3 "Mechanisms of the host immune response and helminth-induced pathology during Trichobilharzia regenti (Schistosomatidae) neuroinvasion in mice". PLOS Pathogens 18 (2): e1010302. February 2022. doi:10.1371/journal.ppat.1010302. PMID 35120185.
- ↑ "Radix spp.: Identification of trematode intermediate hosts in the Czech Republic". Acta Parasitologica 57 (3): 273–84. September 2012. doi:10.2478/s11686-012-0040-7. PMID 22875675.
- ↑ Peštová J (2015). Differentiation of totipotent germinal cells in larvae of bird schistosomes [in Czech] (Master's thesis). Prague, Czech Republic: Charles University.
- ↑ "Changes in surface glycosylation and glycocalyx shedding in Trichobilharzia regenti (Schistosomatidae) during the transformation of cercaria to schistosomulum". PLOS ONE 12 (3): e0173217. 2017-03-15. doi:10.1371/journal.pone.0173217. PMID 28296924. Bibcode: 2017PLoSO..1273217R.
- ↑ 19.0 19.1 19.2 19.3 "Trichobilharzia regenti, a pathogen of the avian and mammalian central nervous systems". Parasitology 119 (6): 577–81. December 1999. doi:10.1017/s0031182099005132. PMID 10633919.
- ↑ 20.0 20.1 20.2 20.3 20.4 "Neurotropic behaviour of Trichobilharzia regenti in ducks and mice". Journal of Helminthology 76 (2): 137–41. June 2002. doi:10.1079/JOH2002113. PMID 12015826.
- ↑ 21.0 21.1 21.2 "Terminal phase of bird schistosomiasis caused by Trichobilharzia regenti (Schistosomatidae) in ducks (Anas platyrhynchos f. domestica)". Folia Parasitologica 54 (2): 105–7. June 2007. doi:10.14411/fp.2007.014. PMID 17886739.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 "Cercarial dermatitis caused by bird schistosomes comprises both immediate and late phase cutaneous hypersensitivity reactions". Journal of Immunology 172 (6): 3766–74. March 2004. doi:10.4049/jimmunol.172.6.3766. PMID 15004181.
- ↑ 23.0 23.1 23.2 "Cercarial dermatitis, a neglected allergic disease". Clinical Reviews in Allergy & Immunology 45 (1): 63–74. August 2013. doi:10.1007/s12016-012-8334-y. PMID 22915284.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 "The severity of mouse pathologies caused by the bird schistosome Trichobilharzia regenti in relation to host immune status". Parasitology Research 93 (1): 8–16. May 2004. doi:10.1007/s00436-004-1079-7. PMID 15034785.
- ↑ 25.0 25.1 25.2 "Trichobilharzia regenti: the developmental differences in natural and abnormal hosts". Parasitology International 54 (3): 167–172. September 2005. doi:10.1016/j.parint.2005.03.003. PMID 15908263.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 "Trichobilharzia regenti: host immune response in the pathogenesis of neuroinfection in mice". Experimental Parasitology 128 (4): 328–35. August 2011. doi:10.1016/j.exppara.2011.04.006. PMID 21554878.
- ↑ 27.0 27.1 "Trichobilharzia regenti (Schistosomatidae): 3D imaging techniques in characterization of larval migration through the CNS of vertebrates". Micron 83: 62–71. April 2016. doi:10.1016/j.micron.2016.01.009. PMID 26897588.
- ↑ "In vitro stimulation of penetration gland emptying by Trichobilharzia szidati and T. regenti (Schistosomatidae) cercariae. Quantitative collection and partial characterization of the products". Parasitology Research 96 (4): 230–41. June 2005. doi:10.1007/s00436-005-1347-1. PMID 15868186.
- ↑ "Comparison of cysteine peptidase activities in Trichobilharzia regenti and Schistosoma mansoni cercariae". Parasitology 134 (Pt 11): 1599–609. October 2007. doi:10.1017/S0031182007002910. PMID 17517170. https://opus.lib.uts.edu.au/bitstream/10453/5909/1/2006015343.pdf.
- ↑ "The functional expression and characterisation of a cysteine peptidase from the invasive stage of the neuropathogenic schistosome Trichobilharzia regenti". International Journal for Parasitology 39 (2): 201–11. January 2009. doi:10.1016/j.ijpara.2008.06.010. PMID 18708063.
- ↑ 31.0 31.1 31.2 "Histopathology of CNS and nasal infections caused by Trichobilharzia regenti in vertebrates". Parasitology Research 87 (8): 644–50. August 2001. doi:10.1007/s004360100431. PMID 11511002.
- ↑ "Multiple cathepsin B isoforms in schistosomula of Trichobilharzia regenti: identification, characterisation and putative role in migration and nutrition". International Journal for Parasitology 35 (8): 895–910. July 2005. doi:10.1016/j.ijpara.2005.02.018. PMID 15950230.
- ↑ Pech V (2013). Peroral infections of birds and mammals with the neuropathogenic fluke Trichobilharzia regenti (Master's thesis). Czech Republic: Charles University.
- ↑ "Antibody response of definitive hosts against antigens of two life stages of the neuropathogenic schistosome Trichobilharzia regenti". Parasites & Vectors 8 (1): 400. July 2015. doi:10.1186/s13071-015-1007-y. PMID 26216102.
- ↑ 35.0 35.1 "The peripheral immune response of mice infected with a neuropathogenic schistosome". Parasite Immunology 42 (6): e12710. June 2020. doi:10.1111/pim.12710. PMID 32145079.
- ↑ "Nitric oxide and cytokine production by glial cells exposed in vitro to neuropathogenic schistosome Trichobilharzia regenti". Parasites & Vectors 9 (1): 579. November 2016. doi:10.1186/s13071-016-1869-7. PMID 27842570.
- ↑ 37.0 37.1 37.2 37.3 "Antibody responses induced by Trichobilharzia regenti antigens in murine and human hosts exhibiting cercarial dermatitis". Parasite Immunology 30 (11–12): 585–95. 2008-11-01. doi:10.1111/j.1365-3024.2008.01059.x. PMID 19067839.
Wikidata ☰ Q3283008 entry
 |