Biology:Y RNA
| Y RNA | |
|---|---|
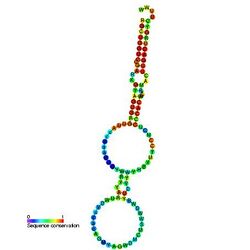 Predicted secondary structure and sequence conservation of Y_RNA | |
| Identifiers | |
| Symbol | Y_RNA |
| Alt. Symbols | Y1; Y3; Y4; Y5 |
| Rfam | RF00019 |
| Other data | |
| RNA type | Gene |
| Domain(s) | Eukaryota |
| SO | 0000405 |
| PDB structures | PDBe |
Y RNAs are small non-coding RNAs. They are components of the Ro60 ribonucleoprotein particle[1] which is a target of autoimmune antibodies in patients with systemic lupus erythematosus.[2] They are also reported to be necessary for DNA replication through interactions with chromatin and initiation proteins.[3][4] However, mouse embryonic stem cells lacking Y RNAs are viable and have normal cell cycles.[5]
Structure
These small RNAs are predicted to fold into a conserved stem formed by the RNA's 3′ and 5′ ends and characterized by a single bulged cytosine, which are the known requirements for Ro binding.[6][7][8]
Function
Two functions have been described for Y RNAs in the literature: As a repressor of Ro60, and as an initiation factor for DNA replication. Mutant human Y RNAs lacking the conserved binding site for Ro60 protein still support DNA replication,[3] indicating that binding to Ro protein and promoting DNA replication are two separable functions of Y RNAs. Although Y RNA-derived small RNAs are similar in size to microRNAs, it has been shown that these Y RNA fragments are not involved in the microRNA pathway.[9]
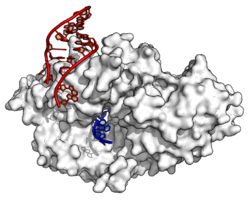
Ro60 Inhibition
In its free state, Ro binds to a variety of misfolded RNAs including misfolded 5S rRNAs, and is thought to act as some sort of quality control mechanism.[10] Crystal structures of Ro complexed either with Y RNA or another RNA showed that Ro binds single-stranded 3′ ends of RNAs relatively nonspecifically, whereas Y RNA binds specifically at a second site that regulates access of other RNAs.[6] In Deinococcus, free Ro has also been shown to function in 23S rRNA maturation.[11] In Deinococcus, mutants lacking Y RNA are viable, and Y RNA appears to be unstable except when complexed with Ro.[11]
DNA replication initiation
Human Y RNAs are functionally required for DNA replication.[3] Biochemical fractionation and reconstitution experiments have established a functional requirement of human Y RNAs for chromosomal DNA replication in isolated vertebrate cell nuclei in vitro[3] and specific degradation of human Y RNAs inhibits DNA replication in vitro, and in intact cells in vivo.[3] Y RNA function is thought to be mediated via interactions with chromatin and initiation proteins (including the origin recognition complex)[4]
In human pathology
Y RNAs are overexpressed in some human tumours and are required for cell proliferation[12] and small, microRNA-sized breakdown products may be involved in autoimmunity and other pathological conditions.[13] Recent work has demonstrated that Y RNAs are modified at their 3′ end by the non-canonical poly(A) polymerase PAPD5, and the short oligo(A) tail added by PAPD5 is a marker for 3′ end processing by the ribonuclease PARN/EXOSC10 or for degradation by the exonuclease DIS3L.[14] Since PARN deficiency causes a severe form of the bone marrow disease Dyskeratosis Congenita as well as pulmonary fibrosis,[15][16] it is possible that defects in Y RNA processing contribute to the severe pathology observed in these patients.
Species distribution
Presumptive Y RNA and Ro protein homologs have been found in eukaryotes and bacteria.[7][17]
Humans
Humans appear to have four Y RNAs, named hY1, hY3, hY4 and hY5[17] and also a large number of pseudogenes.
C. elegans
Caenorhabditis elegans has one, named CeY RNA and a large number of sbRNAs that are postulated to also be Y RNA homologues.[18][19]
D. radiodurans
The radiation-resistant bacterium Deinococcus radiodurans encodes a homolog of Ro called rsr ("Ro sixty related"), and at least four small RNAs accumulate in Deinococcus under conditions where rsr expression is induced (UV irradiation); one of these RNAs appears to be a Y RNA homolog.[20] In Deinococcus radiodurans Rsr is tethered via Y RNA to the exoribonuclease PNPase and channels single-stranded RNA into the PNPase cavity. Rsr and Y RNA enhance degradation of structured RNAs by PNPase. This role could be conserved, as Rsr and ncRNAs called YrlA and YrlB (Y RNA like) also associate with PNPase in an evolutionary distant bacterium Salmonella Typhimurium.[21]
References
- ↑ "Y RNAs: recent developments". Biomolecular Concepts 4 (2): 103–110. April 2013. doi:10.1515/bmc-2012-0050. PMID 25436569.
- ↑ "Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus". Science 211 (4480): 400–402. January 1981. doi:10.1126/science.6164096. PMID 6164096. Bibcode: 1981Sci...211..400L.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Functional requirement of noncoding Y RNAs for human chromosomal DNA replication". Molecular and Cellular Biology 26 (18): 6993–7004. September 2006. doi:10.1128/MCB.01060-06. PMID 16943439.
- ↑ 4.0 4.1 "Dynamic interaction of Y RNAs with chromatin and initiation proteins during human DNA replication". Journal of Cell Science 124 (Pt 12): 2058–2069. June 2011. doi:10.1242/jcs.086561. PMID 21610089.
- ↑ "Noncoding Y RNAs regulate the levels,subcellular distribution and protein interactions of their Ro60 autoantigen partner". Nucleic Acids Research 48 (12): 6919–6930. July 2020. doi:10.1093/nar/gkaa414. PMID 32469055.
- ↑ 6.0 6.1 6.2 "Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity". Cell 121 (4): 529–539. May 2005. doi:10.1016/j.cell.2005.03.009. PMID 15907467.
- ↑ 7.0 7.1 "Conserved features of Y RNAs: a comparison of experimentally derived secondary structures". Nucleic Acids Research 28 (2): 610–619. January 2000. doi:10.1093/nar/28.2.610. PMID 10606662.
- ↑ "Binding of the 60-kDa Ro autoantigen to Y RNAs: evidence for recognition in the major groove of a conserved helix". RNA 4 (7): 750–765. July 1998. doi:10.1017/S1355838298971667. PMID 9671049.
- ↑ "Biogenesis of Y RNA-derived small RNAs is independent of the microRNA pathway". FEBS Letters 586 (8): 1226–1230. April 2012. doi:10.1016/j.febslet.2012.03.026. PMID 22575660.
- ↑ "Emerging themes in non-coding RNA quality control". Current Opinion in Structural Biology 17 (2): 209–214. April 2007. doi:10.1016/j.sbi.2007.03.012. PMID 17395456.
- ↑ 11.0 11.1 "An ortholog of the Ro autoantigen functions in 23S rRNA maturation in D. radiodurans". Genes & Development 21 (11): 1328–1339. June 2007. doi:10.1101/gad.1548207. PMID 17510283.
- ↑ "Noncoding human Y RNAs are overexpressed in tumours and required for cell proliferation". British Journal of Cancer 98 (5): 981–988. March 2008. doi:10.1038/sj.bjc.6604254. PMID 18283318.
- ↑ "Are the Ro RNP-associated Y RNAs concealing microRNAs? Y RNA-derived miRNAs may be involved in autoimmunity". BioEssays 33 (9): 674–682. September 2011. doi:10.1002/bies.201100048. PMID 21735459.
- ↑ "PARN Modulates Y RNA Stability and Its 3′-End Formation". Molecular and Cellular Biology 37 (20). October 2017. doi:10.1128/MCB.00264-17. PMID 28760775.
- ↑ "Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening". Nature Genetics 47 (5): 512–517. May 2015. doi:10.1038/ng.3278. PMID 25848748.
- ↑ "Poly(A)-specific ribonuclease deficiency impacts telomere biology and causes dyskeratosis congenita". The Journal of Clinical Investigation 125 (5): 2151–2160. May 2015. doi:10.1172/JCI78963. PMID 25893599.
- ↑ 17.0 17.1 "Ro-associated Y RNAs in metazoans: evolution and diversification". Molecular Biology and Evolution 24 (8): 1678–1689. August 2007. doi:10.1093/molbev/msm084. PMID 17470436.
- ↑ "Caenorhabditis elegans embryos contain only one major species of Ro RNP". RNA 1 (3): 293–303. May 1995. PMID 7489501.
- ↑ "Nematode sbRNAs: homologs of vertebrate Y RNAs". Journal of Molecular Evolution 70 (4): 346–358. April 2010. doi:10.1007/s00239-010-9332-4. PMID 20349053. Bibcode: 2010JMolE..70..346B.
- ↑ "Ro ribonucleoproteins contribute to the resistance of Deinococcus radiodurans to ultraviolet irradiation". Genes & Development 14 (7): 777–782. April 2000. doi:10.1101/gad.14.7.777. PMID 10766734.
- ↑ "An RNA degradation machine sculpted by Ro autoantigen and noncoding RNA". Cell 153 (1): 166–177. March 2013. doi:10.1016/j.cell.2013.02.037. PMID 23540697.
External links
 |
