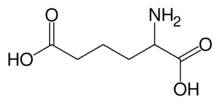
Chemistry:Α-Aminoadipic acid
| |
| Names | |
|---|---|
| IUPAC name
2-Aminohexanedioic acid
| |
| Other names
2-Aminoadipic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1724349 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| MeSH | 2-Aminoadipic+Acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H11NO4 | |
| Molar mass | 161.156 g/mol |
| Appearance | Crystalline |
| Density | 1.333 g/mL |
| Melting point | 196 °C (385 °F; 469 K) |
| Boiling point | 364 °C (687 °F; 637 K) |
| Hazards | |
| Main hazards | Irritant |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P272, P280, P302+352, P321, P333+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-Aminoadipic acid is one of the metabolic precursor in the biosynthesis of lysine through α-aminoadipate pathway. Its conjugate base is α-aminoadipate, which is the prevalent form at physiological pH.
α-Aminoadipic acid has a stereogenic center and can appear in two enantiomers, L-α-aminoadipate and D-α-aminoadipate. The L-enantiomer appears during lysine biosynthesis and degradation, whereas the D-enantiomer is a part of certain antibiotics.
Metabolism
Lysine degradation
Through saccharopine and allysine, lysine is converted to α-aminoadipate, which is then degraded all the way to acetoacetate.[2] Allysine is oxidized by aminoadipate-semialdehyde dehydrogenase:[2]
- allysine + NAD(P)+ ↔ α-aminoadipate NAD(P)H + H+
α-Aminoadipate is then transaminated with α-ketoglutarate to give α-ketoadipate and glutamate, respectively, by the action of 2-aminoadipate transaminase:[2]
- α-aminoadipate + α-ketoglutarate ↔ α-ketoadipate + glutamate
Lysine biosynthesis
α-Aminoadipate appears during biosynthesis of lysine in several yeast species, fungi, and certain protists.[3][4][5][6] During this pathway, which is named after α-aminoadipate, the same steps are repeated in the opposite order as in the degradation reactions, namely, α-ketoadipate is transaminated to α-aminoadipate, which is then reduced to allysine, allysine couples with glutamate to give saccharopine, which is then cleaved to give lysine.[7]
Importance
A 2013 study identified α-aminoadipate as a novel predictor of the development of diabetes and suggested that it is a potential modulator of glucose homeostasis in humans.[8]
D-α-Aminoadipic acid is a part of the antibiotic cephalosporin C.[9]
References
- ↑ "2-Aminohexanedioic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/469#section=Safety-and-Hazards.
- ↑ 2.0 2.1 2.2 Voet, Donald; Voet, Judith G. (2011). Biochemistry (4th ed.). Hoboken, NJ: Wiley. pp. 1040—1041. ISBN 978-0-470-91745-9.
- ↑ "The alpha-aminoadipate pathway for lysine biosynthesis in fungi". Cell Biochemistry and Biophysics 46 (1): 43–64. 2006. doi:10.1385/CBB:46:1:43. PMID 16943623.
- ↑ "Kinetic mechanism of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae". Biochemistry 43 (37): 11790–11795. September 2004. doi:10.1021/bi048766p. PMID 15362863.
- ↑ "alpha-Aminoadipate pathway for the biosynthesis of lysine in lower eukaryotes". Critical Reviews in Microbiology 12 (2): 131–151. 1985. doi:10.3109/10408418509104427. PMID 3928261.
- ↑ "Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant". The Journal of Biological Chemistry 242 (10): 2542–2546. May 1967. doi:10.1016/S0021-9258(18)95997-1. PMID 6026248.
- ↑ "The α-aminoadipate pathway for lysine biosynthesis in fungi". Cell Biochemistry and Biophysics 46 (1): 43–64. 2006. doi:10.1385/CBB:46:1:43. PMID 16943623.
- ↑ "2-Aminoadipic acid is a biomarker for diabetes risk". Journal of Clinical Investigation 123 (10): 4309–4317. 2013. doi:10.1172/JCI64801. PMID 24091325.
- ↑ Newton, G. G. F.; Abraham, E. P. (1955). "Cephalosporin C, a New Antibiotic containing Sulphur and D-α-Aminoadipic Acid" (in en). Nature 175 (4456): 548. doi:10.1038/175548a0. ISSN 1476-4687. PMID 14370161. Bibcode: 1955Natur.175..548N. https://www.nature.com/articles/175548a0.
 |

