Chemistry:1,1,1,3,3,3-Hexachloropropane
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,1,3,3,3-Hexachloropropane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H2Cl6 | |
| Molar mass | 250.77 g/mol |
| Density | d204 1.68 g/mL |
| Melting point | −27 °C[1][2] |
| Boiling point | 206 °C (760 torr),[1][2] 114-124 °C (20 torr),[3] 89 °C (16 torr)[1][2] |
Refractive index (nD)
|
n20D 1.5179 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H315, H319, H332, H335, H400 | |
| P261, P264, P271, P273, P280, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
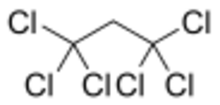
1,1,1,3,3,3-Hexachloropropane is a compound of chlorine, hydrogen, and carbon, with chemical formula C
3Cl
6H
2, specifically Cl
3C–CH
2–CCl
3. Its molecule can be described as that of propane with chlorine atoms substituted for the six hydrogen atoms on the extremal carbons.[4]
History and properties
There are 29 chlorinated derivatives of propane (four of them being hexachloropropanes, with the formula C
3Cl
6H
2). This was the last one of them to be synthesized—by A. W. Davis and A. M. Whaley—in 1950.[2]
1,1,1,3,3,3-Hexachloropropane is a liquid that boils at 206 °C.[1][2] Its boiling point is significantly higher than expected based on estimations from various molecular parameters.[5][6][7]
Production
The original synthesis by Davis and Whaley obtained the compound by reacting 1,1,3,3-tetrachloropropane and/or 1,1,1,3-tetrachloropropane with chlorine at 80-100 °C, through 1,1,1,3,3-pentachloropropane as an intermediate step.[2]
The compound can be produced quantitatively also by reacting carbon tetrachloride CCl
4 and 1,1-dichloroethene Cl
2C=CH
2 at 80-150 °C, with a copper-based catalyst, such as copper(I) chloride or copper(II) chloride, and possibly an amine as co-catalyst.[3][8][9][10][11][12][13][14] The same process can generate higher chlorinated alkanes of the form H
3C–(CH
2–CCl
2)
nCl.[3][11]
Applications
The compound has been considered as an intermediate in the manufacture of 1,1,1,3,3,3-hexafluoropropane, through reaction with hydrogen fluoride.[15][16][17][18]
Safety
A 2001 study found that the compound had significant effects on rat fetuses when inhaled at 25 ppm. The LD50 (injection, rats) was found to be 827 mg/kg.[19][20]
See also
- 1,1,2,2,3,3-Hexachloropropane
- 1,1,1,3,3,3-Hexafluoropropane
- Octachloropropane
- 1,1,1,3,3,3-Hexachloro-2,2-difluoropropane
References
- ↑ 1.0 1.1 1.2 1.3 1.4 US National Institutes of Health (2020): "1,1,1,3,3,3-Hexachloropropane". Compound page at the PubChem online database, NCBI. Accessed on 2020-07-14.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 H. W. Davis and A. M. Whaley (1950): "Preparation of 1,1,1,3,3,3-hexachloropropane". Journal of the American Chemical Society, volume 73, issue 5, page 2361. doi:10.1021/ja01149a514
- ↑ 3.0 3.1 3.2 Meir Asscher, Aharon Katchalsky, and David Vofsi (1961): "Production of adducts of carbon tetrachloride or chloroform with olefinically unsaturated substances". US Patent 3651019. Granted on 1972-03-21, assigned to Yeda Research and Development; expired on 1989-03-21.
- ↑ United States Environmental Protection Agency (2020): "Propane, 1,1,1,3,3,3-hexachloro-". Compound page at the DSSTox online database. Accessed on 2020-07-14.
- ↑ Alexandru T. Balaban, Subhash C. Basak, Timothy Colburn, and Gregory D. Grunwald (1994): "Correlation between structure and normal boiling points of haloalkanes C1-C4 using neural networks". Journal of Chemical Information and Computer Science, volume 34, issue 5, pages 1118–1121. doi:10.1021/ci00021a016
- ↑ Subhash C. Basak, Brian D. Gute, and Gregory D. Grunwald (1996): "Estimation of the normal boiling points of haloalkanes using molecular similarity". Croatia Chemica Acta, volume 69, issue 3, pages 1159-1173.
- ↑ Subhash C. Basak, Brian D. Gute, and Gregory D. Grunwald (1996): "A comparative study of topological and geometrical parameters in estimating normal boiling point and octanol/water partition coefficient". Journal of Chemical Information and Computer Science, volume 36, issue 6, pages 1054–1060. doi:10.1021/ci960024i
- ↑ Teruzo Asahara, Manabu Senō, and Cheng-Ching Wu (1969): "Telomerization of ethylene with carbon tetrachloride initiated by amine-cupric chloride complexes". Kogyo Kagaku Zasshi (Journal of Industrial Chemistry; Journal of the Chemical Society of Japan, Industrial Chemistry Section), volume 72, pages 1822-1824.
- ↑ Mohamed Belbachir, Bernard Boutevin, Yves Piétrasanta, and Gérard Rigal (1984) "Télomérisation du chlorure de vinylidène, 1. Réaction avec le tétrachlorure de carbone par catalyse rédox". Makromolekulare Chemie, volume 185, issue 8, pages 1583-1595. doi:10.1002/macp.1984.021850808
- ↑ Mohamed Belbachir, Bernard Boutevin, Yves Piétrasanta, and Gérard Rigal (1984): "Télomérisation du chlorure de vinylidène, 2. Réaction avec des télogènes du type R-CCL3 et préparation de composés dérivés". Makromolekulare Chemie (Macromolecular Chemistry and Physics), volume 185, issue 8, pages 1597-1606. doi:10.1002/macp.1984.021850809
- ↑ 11.0 11.1 Martin Kotora and Milan Hájek (1991): "Selective additions of polyhalogenated compounds to chloro substituted ethenes catalyzed by a copper complex". Reaction Kinetics and Catalysis Letters, volume 44, pages 415–419. doi:10.1007/BF02073008
- ↑ Richard Leroy Wilson, Charles Richard Cupit, and Rodney Lee Klausmeyer (1996): "Method for the manufacture of 1,1,1,3,3,3-hexachloropropane". US Patent 5792893. Granted on 1998-08-11, Assigned to Vulcan Materials, then Basic Chemicals, then Occidental Chemical; expired on 2016-07-09.
- ↑ 韩箴贤 Hán Xhēnxián, 徐卫国 Xú Wèiguó, 尤来方 Yóu Láifāng, 吴江平 Wú Jiāngpíng (1998): "1,1,1,3,3,3-六氯丙烷制备方法" ("1,1,1,3,3,3-hexachloropropane preparation method"). Chinese patent 1218788A. Granted on 1999-06-09.
- ↑ 蒋琦 Jiǎng Qí (2002): "1,1,1,3,3,3-六氯丙烷的制备方法" ("Preparation method of 1,1,1,3,3,3-hexachloropropane"). Chinese patent 1150145C. Granted on 2004-05-19.
- ↑ Pennetreau, Pascal (1992): "Process for the preparation of 1-chloro-1,1,3,3,3-pentafluoropropane and of 1,1,1,3,3,3-hexafluoropropane". French patent EP 0522639 (A1). Granted on 1993-01-13; assigned to Solvay.
- ↑ Michael Van Der Puy, G. V. Bindu Madhavan, and Timothy R. Demmin (1993): "Process for the preparation of hydrofluorocarbons having 3 to 7 carbon atoms" US Patent 5395997. Granted on 1995-03-07, assigned to AlliedSignal then Honeywell International; expired on 2013-07-29.
- ↑ N. Mallikarjuna Rao (1994): "Process for the manufacture of 1,1,1,3,3,3-hexafluoropropane". US Patent 5545774. Granted on 1996-08-13, assigned to DuPont; expired on 2014-12-08.
- ↑ Kevin Robert Benson, David Elliott Bradley, and David Nalewajek (). "Continuous production of 1,1,1,3,3,3-hexafluoropropane and 1-chloro-1,1,3,3,3-pentafluoropropane" US Patent 5811604. Granted on 1998-09-22; assigned to AlliedSignal, then Honeywell International. Expired on 2017-02-05.
- ↑ J. R. Bamberger, G. S. Ladics, M. E. Hurtt, M. S. Swanson, and W. J. Brock (2001): "Subchronic inhalation toxicity of the chlorinated propane 1,1,1,3,3,3-hexachloropropane (HCC-230fa) in rats". Toxicological Sciences, volume 62, issue 1, pages 155-165. doi:10.1093/toxsci/62.1.155. PMID 11399803
- ↑ W. J. Brock, S. M. Munley, M. S. Swanson, K. M. McGown, M. E. Hurtt (2003): "Developmental toxicity and genotoxicity studies of 1,1,1,3,3,3-hexachloropropane (HCC-230fa) in rats". Toxicological Sciences, volume 75, issue 2, pages 448–457. doi:10.1093/toxsci/kfg193
 |

