Chemistry:1,2-Dioxolane
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dioxolane | |
| Systematic IUPAC name
1,2-Dioxacyclopentane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.079 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
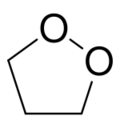
1,2-Dioxolane is a chemical compound with formula C3H6O2, consisting of a ring of three carbon atoms and two oxygen atoms in adjacent positions. Its condensed structural formula is [–(CH2)3–O–O–].[1][2]
The compound is an organic peroxide, specifically an endoperoxide, and a structural isomer of the much more common 1,3-dioxolane, which is often called simply "dioxolane".
Synthesis
Synthesis methods for the 1,2-dioxolane core structure include oxidation of cyclopropane derivatives with singlet oxygen[3] or molecular oxygen with a suitable catalyst,[4][5] the use of autooxidation, nucleophilic displacement with hydrogen peroxide, treatment with mercury(II) nitrate, photolysis of extended π-systems,[6] reaction of a bis-silylperoxide and an alkene,[7] or reaction with a 2-perhydroxy 4-alkene with diethylamine[8] or mercury(II) acetate.[9][10]
Occurrence
Some derivatives occur naturally, for example in Calophyllum dispar and from the seeds of the mamey (Mammea americana).[6] Plakinic acid A (3,5-peroxy 3Z,5Z,7,11-tetramethyl 13-phenyl-8E,12E-tridecadienoic acid) and similar compounds were isolated from sponges of the Plakortis genus.[11][12] Nardosinone is a sesquiterpene derivative with a 1,2-dioxolane element isolated from the plant Adenosma caeruleum.[13]
Uses
Synthetic and natural dioxolane derivatives have been used or considered as antimalarial drugs.[6][7] Plakinic acid A and related compounds showed antifungal action.[11]
See also
References
- ↑ "Enthalpies of formation of cyclic alkyl peroxides: Dioxirane, 1,2-dioxetane, 1,2-dioxolane, and 1,2-dioxane". Chemical Physics Letters 268 (1–2): 175–179. 199. doi:10.1016/S0009-2614(97)00168-1. Bibcode: 1997CPL...268..175L.
- ↑ "2.5.2.5 Elements with two or three internal rotors". Diamagnetic Molecules. Landolt-Börnstein - Group II Molecules and Radicals. 14a. 1982. pp. 415–425. doi:10.1007/10201404_40. ISBN 3-540-11365-7.
- ↑ "Formation of 1,2-dioxolane in the singlet oxygenation of cyclopropane". Tetrahedron Letters 32 (52): 7695–7698. 1991. doi:10.1016/0040-4039(91)80568-Q.
- ↑ "Stereochemical studies on the preparation and subsequent reductive cleavage of 1,2-dioxolanes. Application to the synthesis of (±)-yashabushitriol". Tetrahedron Letters 30 (50): 6985–6988. 1989. doi:10.1016/S0040-4039(01)93404-5.
- ↑ "Zinc-Catalyzed Multicomponent Reactions: Easy Access to Furyl-Substituted Cyclopropane and 1,2-Dioxolane Derivatives". European Journal of Organic Chemistry 2016 (15): 2681–2687. 2016. doi:10.1002/ejoc.201600393. https://ria.asturias.es/RIA/bitstream/123456789/8188/1/Archivo.pdf.
- ↑ 6.0 6.1 6.2 "Synthesis and antimalarial activity of some new 1,2-dioxolane derivatives". Journal of Enzyme Inhibition and Medicinal Chemistry 17 (6): 431–7. December 2002. doi:10.1080/1475636021000005677. PMID 12683680.
- ↑ 7.0 7.1 "Synthesis of spiro-1,2-dioxolanes and their activity against Plasmodium falciparum". Bioorganic & Medicinal Chemistry Letters 18 (24): 6521–4. December 2008. doi:10.1016/j.bmcl.2008.10.083. PMID 18993067.
- ↑ "Synthesis and cleavage studies of a 1,2-dioxolane-type peroxide". Chinese Journal of Chemistry 22 (9): 1029–1033. 2010. doi:10.1002/cjoc.20040220930.
- ↑ "1,2,4-Trioxane versus 1,2-dioxolane formation in the mercury(II) acetate-mediated cyclisation of hemiperoxyacetals derived from allylic hydroperoxides". Journal of the Chemical Society, Chemical Communications (5): 428. 1992. doi:10.1039/C39920000428.
- ↑ "A short synthesis of naturally occurring and other analogues of plakinic acids that contain the 1,2-dioxolane group A short synthesis of naturally occurring and other analogues of plakinic acids that contain the 1,2-dioxolane group". Tetrahedron Letters 37 (11): 1885–1888. 1996. doi:10.1016/0040-4039(96)00143-8.
- ↑ 11.0 11.1 "Antifungal peroxide-containing acids from two Caribbean sponges". Journal of the American Chemical Society 105 (26): 7735–7736. 1983. doi:10.1021/ja00364a045.
- ↑ "Asymmetric synthesis of 1,2-dioxolane-3-acetic acids: synthesis and configurational assignment of plakinic acid A". The Journal of Organic Chemistry 71 (6): 2283–92. March 2006. doi:10.1021/jo0522254. PMID 16526775.
- ↑ "Synthesis and reactivity of 1,2-dioxolanes from β,γ-epoxy ketones". Organic Letters 16 (10): 2650–3. May 2014. doi:10.1021/ol500835f. PMID 24779430.
 |

