Chemistry:2,4,6-Trichlorobenzoyl chloride
| |
| Names | |
|---|---|
| IUPAC name
2,4,6-trichlorobenzoyl chloride
| |
| Other names
Yamaguchi's reagent
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | TCBC |
| 2050280 | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3265 |
| |
| |
| Properties | |
| C7H2Cl4O | |
| Molar mass | 243.89 g·mol−1 |
| Appearance | Light yellow liquid |
| Density | 1.561 g/mL |
| Boiling point | 107 - 108 °C (225 - 226 °F) |
| Reacts with water | |
| log P | 2.738 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P264, P280, P301+330+331, P303+361+353, P304+340+310, P305+351+338+310, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 113 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,4,6-Trichlorobenzoyl chloride or Yamaguchi's reagent is an chlorinated aromatic compound that is commonly used in a variety of organic syntheses.[2][3]
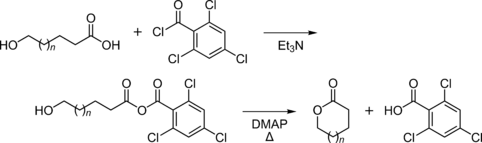
Yamaguchi esterification
It is the primary reactant in Yamaguchi esterification. 2,4,6-Trichlorobenzoyl chloride readily reacts with alcohols. This newly formed reagent, when mixed with a stoichiometric amount of 4-dimethylaminopyridine, cyclizes and forms esters. This reaction creates 2,4,6-trichlorobenzoic acid as a byproduct.
Preparation
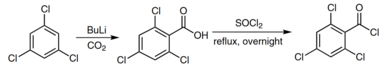
2,4,6-Trichlorobenzoyl chloride is prepared by reacting 2,4,6-trichloroaniline with N-butyllithium in a carbon dioxide atmosphere. This produces 2,4,6-trichlorobenzoic acid, which can then be refluxed in thionyl chloride to form 2,4,6-trichlorobenzoyl chloride.[4]
Since 2,4,6-trichlorobenzoic acid is produced as a by product of the esterification process, it can be refluxed again to recreate 2,4,6-trichlorobenzoyl chloride.
References
- ↑ "2,4,6-Trichlorobenzoyl chloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/2733703#section=Safety-and-Hazards.
- ↑ Fürstner, Alois; Fasching, Bernhard; O'Neil, Gregory W.; Fenster, Michaël D. B.; Godbout, Cédrickx; Ceccon, Julien (2007). "Toward the total synthesis of spirastrellolide A. Part 3: Intelligence gathering and preparation of a ring-expanded analogue" (in en). Chem. Commun. (29): 3045–3047. doi:10.1039/B707835H. ISSN 1359-7345. PMID 17639136. http://xlink.rsc.org/?DOI=B707835H.
- ↑ Panek, J. S., ed (2007) (in en). Category 3, Compounds with Four and Three Carbon Heteroatom Bonds: Three Carbon—Heteroatom Bonds: Esters, and Lactones; Peroxy Acids and R(CO)OX Compounds; R(CO)X, X=S, Se, Te. Stuttgart: Georg Thieme Verlag. doi:10.1055/sos-sd-020-01369. ISBN 978-3-13-144691-6. http://www.thieme-connect.de/DOI/DOI?10.1055/b-003-125722.[yes|permanent dead link|dead link}}]
- ↑ Kotammagari, Tharun (2014-04-28). "2,4,6-Trichlorobenzoyl Chloride (Yamaguchi Reagent)" (in en). Synlett 25 (9): 1335–1336. doi:10.1055/s-0033-1341245. ISSN 0936-5214.
 |