Chemistry:2,6-Diaminopurine
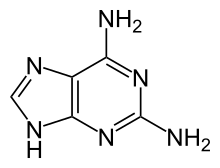
| |
| Names | |
|---|---|
| IUPAC name
7H-purine-2,6-diamine
| |
| Other names
2-aminoadenine; 2,6-DAP
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H6N6 | |
| Molar mass | 150.145 g·mol−1 |
| Appearance | White to yellow crystalline powder |
| Density | 1.743 g/cm3 |
| Melting point | 117 to 122 °C (243 to 252 °F; 390 to 395 K) |
| 2.38 g/L at 20 °C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,6-diaminopurine (2,6-DAP, also known as 2-aminoadenine) is a compound once used in the treatment of leukemia.[1] As the Z base, it is found instead of adenine (A) in the genetic material of some bacteriophage viruses.[2]
In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting 2,6-diaminopurine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space.[3][4][5]
In viruses
In cyanophage S-2L (Siphoviridae), diaminopurine is used instead of adenine (host evasion).[6] Diaminopurine base (Z) pairs perfectly with thymine (T) as it is identical to adenine (A) but has an amine group at position 2 forming 3 intermolecular hydrogen bonds, eliminating the major difference between the two types of basepairs (weak:A-T and strong:C-G). This improved stability affects protein-binding interactions that rely on those differences.
Four papers published April 2021 further describes the use and production of the Z-base. It is now known that:[7]
- The S-2L phage avoids incorporating A bases in the genome by hydrolyzing dATP (DatZ enzyme);[8]
- The Z base is produced by a pathway involving DUF550 (MazZ) and PurZ in S-2L and Vibrio phage PhiVC8;[9]
- The PrimPol/AEP DNA polymerase responsible for handling the Z base occurs in the same gene cluster as the three aforementioned enzymes;[10]
- The Z base is quite widespread in both Siphoviridae and Podoviridae, based on the occurrence of the said gene cluster.[11]
In August 2021, it was shown that DatZ, MazZ and PurZ are sufficient to replace some occurrence of A by Z in the bacterial genome of E. coli; expression of this system is toxic to the cell. The structures of MazZ (subtype 2) and PurZ are also determined, showing a possible link between PurZ and archaeal versions of PurA.[12]
Biosynthesis
2-aminoadenine is produced in two steps. The enzyme MazZ (homologous to MazG, EC 3.6.1.8) first performs:[12]
- dGTP + H2O = dGMP + diphosphate
The enzyme PurZ (homologous to PurA, EC 6.3.4.4) then performs:[9]
- (d)ATP + dGMP + L-aspartate = (d)ADP + phosphate + 2-aminodeoxyadenylosuccinate (dSMP)
The resulting dSMP is processed by host enzymes analogously to adenylosuccinate to produce dZTP.
In cellular life
This article is missing information about results of the altered H-bond strength in DNA and RNA. (October 2021) |
2,6-DAP was used to treat leukemia since as early as 1951.[13] It is known to arrest progression of cell cycle in mouse leukemia cells by 1989.[14] Cancer cells are known to become resistant to DAP by losing their adenine phosphoribosyltransferase (APRT) function,[15] a process shared with E. coli.[16]
DAP derivatives are in vitro antivirals useful against pseudorabies virus, a economically important livestock disease.[17] This base, in its free form, is able to correct UGA nonsense mutations by encouraging translational readthrough, through the inhibition of FTSJ1.[18]
Bioengineering
In bioengineering, anti-miRNA oligonucleotides (specifically, the serinol nucleic acid [SNA] type) incorporating base Z instead of A show enhanced binding to RNA.[19]
DAP is used similar to other nuclear acid analogues in the investigation of enzyme structures and mechanisms.[20]
References
- ↑ "George H. Hitchings". nobelprize.org. https://www.nobelprize.org/nobel_prizes/medicine/laureates/1988/hitchings-bio.html.
- ↑ "Some viruses thwart bacterial defenses with a unique genetic alphabet". 5 May 2021. https://www.sciencenews.org/article/virus-dna-z-bacteriophage-genetic-alphabet-bond-life.
- ↑ Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". Proceedings of the National Academy of Sciences (PNAS) 108 (34): 13995–13998. doi:10.1073/pnas.1106493108. PMID 21836052. Bibcode: 2011PNAS..10813995C.
- ↑ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. http://www.nasa.gov/topics/solarsystem/features/dna-meteorites.html. Retrieved 2011-08-10.
- ↑ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. https://www.sciencedaily.com/releases/2011/08/110808220659.htm. Retrieved 2011-08-09.
- ↑ Kirnos MD, Khudyakov IY, Alexandrushkina NI, Vanyushin BF. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–70.
- ↑ Jacinta Bowler: Some Viruses Have a Completely Different Genome to The Rest of Life on Earth, on: sciencealert, 4 MAY 2021
- ↑ Czernecki, Dariusz; Legrand, Pierre; Tekpinar, Mustafa; Rosario, Sandrine; Kaminski, Pierre-Alexandre; Delarue, Marc (2021-04-23). "How cyanophage S-2L rejects adenine and incorporates 2-aminoadenine to saturate hydrogen bonding in its DNA" (in en). Nature Communications 12 (1): 2420. doi:10.1038/s41467-021-22626-x. ISSN 2041-1723. PMID 33893297. Bibcode: 2021NatCo..12.2420C.
- ↑ 9.0 9.1 Sleiman, Dona; Garcia, Pierre Simon; Lagune, Marion; Loc’h, Jerome; Haouz, Ahmed; Taib, Najwa; Röthlisberger, Pascal; Gribaldo, Simonetta et al. (30 April 2021). "A third purine biosynthetic pathway encoded by aminoadenine-based viral DNA genomes". Science 372 (6541): 516–520. doi:10.1126/science.abe6494. PMID 33926955. Bibcode: 2021Sci...372..516S.
- ↑ Pezo, Valerie; Jaziri, Faten; Bourguignon, Pierre-Yves; Louis, Dominique; Jacobs-Sera, Deborah; Rozenski, Jef; Pochet, Sylvie; Herdewijn, Piet et al. (30 April 2021). "Noncanonical DNA polymerization by aminoadenine-based siphoviruses". Science 372 (6541): 520–524. doi:10.1126/science.abe6542. PMID 33926956. Bibcode: 2021Sci...372..520P.
- ↑ Zhou, Yan; Su, Xuexia; Wei, Yifeng; Cheng, Yu; Guo, Yu; Khudyakov, Ivan; Liu, Fuli; He, Ping et al. (30 April 2021). "A widespread pathway for substitution of adenine by diaminopurine in phage genomes". Science 372 (6541): 512–516. doi:10.1126/science.abe4882. PMID 33926954. Bibcode: 2021Sci...372..512Z. https://www.researchgate.net/publication/351214584.
- ↑ 12.0 12.1 Czernecki, Dariusz; Bonhomme, Frédéric; Kaminski, Pierre-Alexandre; Delarue, Marc (2021-08-05). "Characterization of a triad of genes in cyanophage S-2L sufficient to replace adenine by 2-aminoadenine in bacterial DNA" (in en). Nature Communications 12 (1): 4710. doi:10.1038/s41467-021-25064-x. ISSN 2041-1723. PMID 34354070. Bibcode: 2021NatCo..12.4710C.
- ↑ BURCHENAL, JH; KARNOFSKY, DA; KINGSLEY-PILLERS, EM; SOUTHAM, CM; MYERS, WP; ESCHER, GC; CRAVER, LF; DARGEON, HW et al. (May 1951). "The effects of the folic acid antagonists and 2,6-diaminopurine on neoplastic disease, with special reference to acute leukemia.". Cancer 4 (3): 549–69. doi:10.1002/1097-0142(195105)4:3<549::aid-cncr2820040308>3.0.co;2-j. PMID 14839611.
- ↑ Weckbecker, G; Cory, JG (1989). "Metabolic activation of 2,6-diaminopurine and 2,6-diaminopurine-2'-deoxyriboside to antitumor agents.". Advances in Enzyme Regulation 28: 125–44. doi:10.1016/0065-2571(89)90068-x. PMID 2624171.
- ↑ Shao, C; Deng, L; Henegariu, O; Liang, L; Stambrook, PJ; Tischfield, JA (20 June 2000). "Chromosome instability contributes to loss of heterozygosity in mice lacking p53.". Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7405–10. doi:10.1073/pnas.97.13.7405. PMID 10861008. Bibcode: 2000PNAS...97.7405S.
- ↑ Kocharian, ShM; Chukanova, TI; Sukhodolets, VV (1977). "[Mutations of resistance to 2,6-diaminopurine and 6-methylpurine that affect adenine phosphoribosyltransferase in Escherichia coli K-12].". Genetika 13 (10): 1821–30. PMID 348574.
- ↑ Zouharova, D; Lipenska, I; Fojtikova, M; Kulich, P; Neca, J; Slany, M; Kovarcik, K; Turanek-Knotigova, P et al. (29 February 2016). "Antiviral activities of 2,6-diaminopurine-based acyclic nucleoside phosphonates against herpesviruses: In vitro study results with pseudorabies virus (PrV, SuHV-1).". Veterinary Microbiology 184: 84–93. doi:10.1016/j.vetmic.2016.01.010. PMID 26854349.
- ↑ Trzaska, C; Amand, S; Bailly, C; Leroy, C; Marchand, V; Duvernois-Berthet, E; Saliou, JM; Benhabiles, H et al. (20 March 2020). "2,6-Diaminopurine as a highly potent corrector of UGA nonsense mutations.". Nature Communications 11 (1): 1509. doi:10.1038/s41467-020-15140-z. PMID 32198346. Bibcode: 2020NatCo..11.1509T.
- ↑ Kamiya, Y; Donoshita, Y; Kamimoto, H; Murayama, K; Ariyoshi, J; Asanuma, H (5 October 2017). "Introduction of 2,6-Diaminopurines into Serinol Nucleic Acid Improves Anti-miRNA Performance.". ChemBioChem 18 (19): 1917–1922. doi:10.1002/cbic.201700272. PMID 28748559.
- ↑ Bailly, C (1 October 1998). "The use of diaminopurine to investigate structural properties of nucleic acids and molecular recognition between ligands and DNA". Nucleic Acids Research 26 (19): 4309–4314. doi:10.1093/nar/26.19.4309. PMID 9742229.
 |


