Biology:Adenine phosphoribosyltransferase
 Generic protein structure example |
Adenine phosphoribosyltransferase (APRTase) is an enzyme encoded by the APRT gene, found in humans on chromosome 16.[1] It is part of the Type I PRTase family and is involved in the nucleotide salvage pathway, which provides an alternative to nucleotide biosynthesis de novo in humans and most other animals.[2] In parasitic protozoa such as giardia, APRTase provides the sole mechanism by which AMP can be produced.[3] APRTase deficiency contributes to the formation of kidney stones (urolithiasis) and to potential kidney failure.[4]
Function
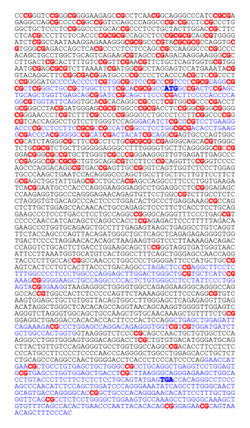
APRTase catalyzes the following reaction in the purine nucleotide salvage pathway:
Adenine + Phosphoribosyl Pyrophosphate (PRPP) → Adenylate (AMP) + Pyrophosphate (PPi)
In organisms that can synthesize purines de novo, the nucleotide salvage pathway provides an alternative that is energetically more efficient. It can salvage adenine from the polyamine biosynthetic pathway or from dietary sources of purines.[2] Although APRTase is functionally redundant in these organisms, it becomes more important during periods of rapid growth, such as embryogenesis and tumor growth.[5] It is constitutively expressed in all mammalian tissue.[6]
In protozoan parasites, the nucleotide salvage pathway provides the sole means for nucleotide synthesis. Since the consequences of APRTase deficiency in humans is comparatively mild and treatable, it may be possible to treat certain parasitic infections by targeting APRTase function.[7]
In plants, as in other organisms, ARPTase functions primarily for the synthesis of adenylate. It has the unique ability to metabolize cytokinins—a plant hormone that can exist as a base, nucleotide, or nucleoside—into adenylate nucleotides.[8]
APRT is functionally related to hypoxanthine-guanine phosphoribosyltransferase (HPRT).
Structure
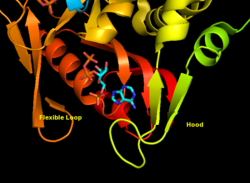
APRTase is a homodimer, with 179 amino acid residues per monomer. Each monomer contains the following regions:
- "Core" domain (residues 33-169) with five parallel β-sheets
- "Hood" domain (residues 5-34) with 2 α-helices and 2 β-sheets
- "Flexible loop" domain (residues 95-113) with 2 antiparallel β-sheets[6]
The core is highly conserved across many PRTases. The hood, which contains the adenine binding site, has more variability within the family of enzymes. A 13-residue motif comprises the PRPP binding region and involves two adjacent acidic residues and at least one surrounding hydrophobic residue.[9]
The enzyme's specificity for adenine involves hydrophobic residues Ala131 and Leu159 in the core domain. In humans, two residues in the hood domain hydrogen bond with the purine for further specificity: Val25 with the hydrogens on N6, and Arg27 with N1. Although the flexible loop does not interact with the hood during purine recognition, it is thought to close over the active site and sequester the reaction from solvents.[6]
Most research on APRTase reports that Mg2+ is essential for phosphoribosyl transfer, and this is conserved across Type I PRTases.[8] However, a recent effort to resolve the structure of human APRTase was unable to locate a single site for Mg2+, but did find evidence to suggest a Cl− atom near Trp98. Despite the difficulty of placing Mg2+, it is generally accepted that the catalytic mechanism is dependent on this ion.[2]
Mechanism
APRTase proceeds via a bi bi ordered sequential mechanism, involving the formation of a ternary complex. The enzyme first binds PRPP, followed by adenine. After the phosphoribosyl transfer occurs, pyrophosphate leaves first, followed by AMP. Kinetic studies indicate that the phosphoribosyl transfer is relatively fast, while the product release (particularly the release of AMP) is rate-limiting.[5]
In human APRTase, it is thought that adenine's N9 proton is abstracted by Glu104 to form an oxacarbenium transition state. This functions as the nucleophile to attack the anomeric carbon of PRPP, forming AMP and displacing pyrophosphate from PRPP. The mechanism of APRTase is generally consistent with that of other PRTases, which conserve the function of displacing PRPP's α-1-pyrophosphate using a nitrogen nucleophile, in either an SN1 or SN2 attack.[2]
Deficiency
When APRTase has reduced or nonexistent activity, adenine accumulates from other pathways. It is degraded by xanthine dehydrogenase to 2,8-dihydroxyadenine (DHA). Although DHA is protein-bound in plasma, it has poor solubility in urine and gradually precipitates in kidney tubules, leading to the formation of kidney stones (urolithiasis). If left untreated, the condition can eventually produce kidney failure.[4]
ARPTase deficiency was first diagnosed in the UK in 1976. Since then, two categories of APRTase deficiency have been defined in humans.[10]
Type I deficiency results in a complete loss of APRTase activity and can occur in patients that are homozygous or compound heterozygous for various mutations.[11] Sequencing has revealed many different mutations that can account for Type 1, including missense mutations, nonsense mutations, a duplicated set of 4 base pairs in exon 3,[12] and a single thymine insertion in intron 4.[13] These mutations cause effects that are clustered into three main areas: in the binding of PRPP's β-phosphate, in the binding of PRPP's 5'-phosphate, and in the segment of the flexible loop that closes over the active site during catalysis [6] Type I deficiency has been observed in various ethnic groups but studied predominately among White populations.[13]
Type II deficiency causes APRTase to have a reduced affinity for PRPP, resulting in a tenfold increase in the KM value.[2] It has been observed and studied primarily in Japan .[13]
A diagnosis of APRTase deficiency can be made by analyzing kidney stones, measuring DHA concentrations in urine, or analyzing APRTase activity in erythrocytes. It is treatable with regular doses of allopurinol or febuxostat, which inhibit xanthine dehydrogenase activity to prevent the accumulation and precipitation of DHA.[14] The condition can also be attenuated with a low-purine diet and high fluid intake.[10]
References
- ↑ "Adenine phosphoribosyltransferase (APRT) deficiency: identification of a novel nonsense mutation". BMC Nephrology 15: 102. 1 July 2014. doi:10.1186/1471-2369-15-102. PMID 24986359.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Structural complexes of human adenine phosphoribosyltransferase reveal novel features of the APRT catalytic mechanism". Journal of Biomolecular Structure & Dynamics 25 (6): 589–97. Jun 2008. doi:10.1080/07391102.2008.10507205. PMID 18399692.
- ↑ "The adenine phosphoribosyltransferase from Giardia lamblia has a unique reaction mechanism and unusual substrate binding properties". The Journal of Biological Chemistry 277 (42): 39973–80. Oct 2002. doi:10.1074/jbc.M205595200. PMID 12171924.
- ↑ 4.0 4.1 "Structural analysis of adenine phosphoribosyltransferase from Saccharomyces cerevisiae". Biochemistry 40 (36): 10800–9. Sep 2001. doi:10.1021/bi010465h. PMID 11535055.
- ↑ 5.0 5.1 "Kinetic mechanism of adenine phosphoribosyltransferase from Leishmania donovani". Biochemistry 41 (12): 4020–31. Mar 2002. doi:10.1021/bi0158730. PMID 11900545.
- ↑ 6.0 6.1 6.2 6.3 "Three-dimensional structure of human adenine phosphoribosyltransferase and its relation to DHA-urolithiasis". Biochemistry 43 (24): 7663–71. Jun 2004. doi:10.1021/bi0360758. PMID 15196008.
- ↑ "Closed site complexes of adenine phosphoribosyltransferase from Giardia lamblia reveal a mechanism of ribosyl migration". The Journal of Biological Chemistry 277 (42): 39981–8. Oct 2002. doi:10.1074/jbc.M205596200. PMID 12171925.
- ↑ 8.0 8.1 "Adenine phosphoribosyltransferase isoforms of Arabidopsis and their potential contributions to adenine and cytokinin metabolism". Physiologia Plantarum 115 (1): 56–68. May 2002. doi:10.1034/j.1399-3054.2002.1150106.x. PMID 12010467.
- ↑ "Quantitative structure-activity relationships for calmodulin inhibitors". Chemical & Pharmaceutical Bulletin 38 (8): 2184–9. Aug 1990. doi:10.1248/cpb.38.2184. PMID 2279281.
- ↑ 10.0 10.1 "Diagnosis of adenine phosphoribosyltransferase deficiency as the underlying cause of renal failure in a renal transplant recipient". Nephrology, Dialysis, Transplantation 19 (3): 736–8. Mar 2004. doi:10.1093/ndt/gfg562. PMID 14767036.
- ↑ "Adenine phosphoribosyltransferase deficiency". Clinical Journal of the American Society of Nephrology 7 (9): 1521–7. Sep 2012. doi:10.2215/CJN.02320312. PMID 22700886.
- ↑ "Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients". The Journal of Clinical Investigation 90 (1): 130–5. Jul 1992. doi:10.1172/JCI115825. PMID 1353080.
- ↑ 13.0 13.1 13.2 "Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency". Journal of the American Society of Nephrology 21 (4): 679–88. Apr 2010. doi:10.1681/ASN.2009080808. PMID 20150536.
- ↑ "Adenine Phosphoribosyltransferase Deficiency". SourceGeneReviews. 1993. PMID 22934314.
Further reading
- "Germline and Somatic Mutation at the APRT Locus of Mice and Man". Purine and Pyrimidine Metabolism in Man VIII. Advances in Experimental Medicine and Biology. 370. 1995. pp. 661–4. doi:10.1007/978-1-4615-2584-4_137. ISBN 978-1-4613-6105-3.
- "A case of a compound heterozygote for adenine phosphoribosyltransferase deficiency (APRT*J/APRT*Q0) leading to 2,8-dihydroxyadenine urolithiasis: review of the reported cases with 2,8-dihydroxyadenine stones in Japan". The Journal of Urology 149 (4): 824–6. Apr 1993. doi:10.1016/s0022-5347(17)36222-5. PMID 8455250.
- "Enzymes of the purine interconversion system in chronic lymphatic leukemia: decreased purine nucleoside phosphorylase and adenosine deaminase activity". Blut 39 (5): 309–15. Nov 1979. doi:10.1007/BF01014193. PMID 116697.
- "Adenine phosphoribosyltransferase: a simple spectrophotometric assay and the incidence of mutation in the normal population". Biochemical Genetics 15 (3–4): 265–72. Apr 1977. doi:10.1007/BF00484458. PMID 869896.
- "Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients". The Journal of Clinical Investigation 90 (1): 130–5. Jul 1992. doi:10.1172/JCI115825. PMID 1353080.
- "Identification of a single missense mutation in the adenine phosphoribosyltransferase (APRT) gene from five Icelandic patients and a British patient". American Journal of Human Genetics 49 (6): 1306–11. Dec 1991. PMID 1746557.
- "A mutant allele common to the type I adenine phosphoribosyltransferase deficiency in Japanese subjects". American Journal of Human Genetics 48 (1): 103–7. Jan 1991. PMID 1985452.
- "Polymerase chain reaction amplification and sequence analysis of human mutant adenine phosphoribosyltransferase genes: the nature and frequency of errors caused by Taq DNA polymerase". Mutation Research 249 (1): 169–76. Jul 1991. doi:10.1016/0027-5107(91)90143-C. PMID 2067530.
- "Identification of a splice mutation at the adenine phosphoribosyltransferase locus in a German family". Klinische Wochenschrift 69 (24): 1152–5. Dec 1990. doi:10.1007/BF01815434. PMID 2135300.
- "Crossovers within a short DNA sequence indicate a long evolutionary history of the APRT*J mutation". Human Genetics 85 (6): 600–4. Oct 1990. doi:10.1007/BF00193582. PMID 2227951. https://deepblue.lib.umich.edu/bitstream/2027.42/47628/1/439_2004_Article_BF00193582.pdf.
- "Detection of an amino acid substitution in the mutant enzyme for a special type of adenine phosphoribosyltransferase (APRT) deficiency by sequence-specific protein cleavage". American Journal of Human Genetics 45 (2): 325–31. Aug 1989. PMID 2502918.
- "Human adenine phosphoribosyltransferase deficiency. Demonstration of a single mutant allele common to the Japanese". The Journal of Clinical Investigation 81 (3): 945–50. Mar 1988. doi:10.1172/JCI113408. PMID 3343350.
- "Human adenine phosphoribosyltransferase. Complete amino acid sequence of the erythrocyte enzyme". The Journal of Biological Chemistry 261 (29): 13677–83. Oct 1986. doi:10.1016/S0021-9258(18)67074-7. PMID 3531209.
- "Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement". Proceedings of the National Academy of Sciences of the United States of America 84 (10): 3349–53. May 1987. doi:10.1073/pnas.84.10.3349. PMID 3554238. Bibcode: 1987PNAS...84.3349B.
- "Human adenine phosphoribosyltransferase. Identification of allelic mutations at the nucleotide level as a cause of complete deficiency of the enzyme". The Journal of Clinical Investigation 80 (5): 1409–15. Nov 1987. doi:10.1172/JCI113219. PMID 3680503.
- "Nucleotide sequence of the human APRT gene". Nucleic Acids Research 15 (21): 9086. Nov 1987. doi:10.1093/nar/15.21.9086. PMID 3684585.
- "Analysis of germline and in vivo somatic mutations in the human adenine phosphoribosyltransferase gene: mutational hot spots at the intron 4 splice donor site and at codon 87". Mutation Research 287 (2): 217–25. Jun 1993. doi:10.1016/0027-5107(93)90014-7. PMID 7685481.
- "Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis". Human Molecular Genetics 3 (5): 817–8. May 1994. doi:10.1093/hmg/3.5.817. PMID 7915931.
External links
- Adenine+phosphoribosyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human APRT genome location and APRT gene details page in the UCSC Genome Browser.
 |