Chemistry:2-Ethylhexyl glycidyl ether
| |
| Names | |
|---|---|
| IUPAC name
2-(2-Ethylhexoxymethyl)oxirane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H22O2 | |
| Molar mass | 186.295 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H317, H319, H335 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P272, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P333+313, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
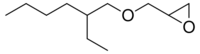
2-Ethylhexyl glycidyl ether is a liquid organic molecule with formula C11H22O2[2] an industrial chemical used to reduce the viscosity of [[epoxy resins.[3] These are then used in adhesives, sealants, and paints or coatings. It has the CAS Registry Number of 2461-15-6.[4][5][6] It has the IUPAC name of 2-(2-ethylhexoxymethyl)oxirane. It also finds use in other polymer based applications.[7]
Synthesis
2-Ethylhexanol and epichlorohydrin are reacted in the presence of a Lewis acid catalyst in a condensation reaction to form a halohydrin. This is followed by a caustic dehydrochlorination, to form 2-ethylhexyl glycidyl ether.[8][9] The waste products are water and sodium chloride and excess caustic soda. One of the quality control tests would involve measuring the Epoxy value by determination of the epoxy equivalent weight.
Commercial
The material is produced domestically in the USA and is also produced in other parts of the world.[10] Over 13 million kg were exported from China in 2019.[11] As well as being used as an epoxy resin diluent, it maybe further reacted to produce cosmetics.[12]
Safety
The safety of the product is fairly well understood. It is classed as a skin sensitizer.[13][14][15][16]
See also
- Allyl glycidyl ether
- n-Butyl glycidyl ether
- Neopentyl glycol diglycidyl ether
- O-Cresyl glycidyl ether (o-CGE)
- Epoxide
- Glycidol
References
- ↑ "2-Ethylhexyl glycidyl ether" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/17162#section=Safety-and-Hazards.
- ↑ "<nowiki>Oxirane, url=https://www.chemeo.com/cid/20-494-1/Oxirane,%20%5B%5B(2-ethylhexyl)oxy%5Dmethyl%5D-" (in en).
- ↑ "TZ3300000 | C11H22O2 | ChemSpider". http://www.chemspider.com/Chemical-Structure.16246.html.
- ↑ "Sigma Aldrich catalogue 2-ethyl hexyl clycidyl ether". https://www.sigmaaldrich.com/US/en/product/aldrich/251747?gclid=CjwKCAjwiuuRBhBvEiwAFXKaNFsWtQCZxQs7zzX3vcT87jBr21i8v6unEHhgTqDm_106N51w973APRoCf8EQAvD_BwE.
- ↑ "2-Ethylhexyl glycidyl ether | 2461-15-6". https://www.chemicalbook.com/ChemicalProductProperty_EN_CB7212453.htm#:~:text=2-Ethylhexyl%20glycidyl%20ether%20Chemical%20Properties,Usage,Production&text=Uses%20It%20constitutes%20a%20reactive,as%20a%20commercial%20reactive%20diluent..
- ↑ 2-ethylhexyl glycidyl ether - Wikidata
- ↑ Deralia, Parveen Kumar; du Poset, Aline Maire; Lund, Anja; Larsson, Anette; Ström, Anna; Westman, Gunnar (2021-04-19). "Oxidation Level and Glycidyl Ether Structure Determine Thermal Processability and Thermomechanical Properties of Arabinoxylan-Derived Thermoplastics". ACS Applied Bio Materials 4 (4): 3133–3144. doi:10.1021/acsabm.0c01550. PMID 35014401. https://doi.org/10.1021/acsabm.0c01550.
- ↑ "Glycidyl 2-Ethylhexyl Ether 2461-15-6", Sax's Dangerous Properties of Industrial Materials (Hoboken, NJ, USA: John Wiley & Sons, Inc.), 2004-10-15, doi:10.1002/0471701343.sdp13115, ISBN 0471701343, http://dx.doi.org/10.1002/0471701343.sdp13115, retrieved 2022-03-23
- ↑ SpadŁo, M. & Iwański, L. & Pokorska, Z.. (2004). The effect of catalyst type on the synthesis of 2-ethylhexyl glycidyl ether. Przemysl Chemiczny. 83. 133-136.
- ↑ Chem, A. A. L.. "ME 102" (in en). https://www.aalchem.com/en/product/category/diluent-dilu/2-ethylhexyl-glycidyl-ether-me-102.
- ↑ "What Is 2-Ethylhexyl Glycidylether, Cas No 2461-15-6 Guide". https://www.echemi.com/products/pid_Seven17560-2-ethylhexylglycidylether.html.
- ↑ Office, European Patent. "European publication server" (in en). https://data.epo.org/publication-server/document?iDocId=5999858&iFormat=0.
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.017.776.
- ↑ "2-Ethylhexyl glycidyl ether (cas 2461-15-6) SDS(Safety Data Sheet) /MSDS download". https://www.guidechem.com/msds/2461-15-6.html.
- ↑ "NIOSHTIC-2 Publications Search - 00188271 - Information profiles on potential occupational hazards: epoxy compounds (non-cyclic).". https://www.cdc.gov/niosh/nioshtic-2/00188271.html.
- ↑ Canada, Environment and Climate Change (2020-08-07). "Screening assessment - Epoxides and Glycidyl Ethers Group". https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/screening-assessment-epoxides-glycidyl-ethers-group.html.
Further reading
- Epoxy resin technology.. Paul F. Bruins, Polytechnic Institute of Brooklyn. New York: Interscience Publishers. 1968. ISBN 0-470-11390-1. OCLC 182890. https://www.worldcat.org/oclc/182890.
- Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542. https://www.worldcat.org/oclc/915134542.
- Lee, Henry (1967). Handbook of epoxy resins. Kris Neville ([2nd, expanded work] ed.). New York: McGraw-Hill. ISBN 0-07-036997-6. OCLC 311631322. https://www.worldcat.org/oclc/311631322.
- "Dow Epoxy Resins". http://nmt.edu/academics/mtls/faculty/mccoy/docs2/chemistry/DowEpoxyResins.pdf.
External Websites

