Chemistry:2-Ethylhexanol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethylhexan-1-ol[1] | |
| Other names
isooctyl alcohol, 2-ethylhexanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1719280 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | 2-ethylhexanol |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18O | |
| Molar mass | 130.231 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 833 mg mL−1 |
| Melting point | −76 °C (−105 °F; 197 K) |
| Boiling point | 180 to 186 °C; 356 to 367 °F; 453 to 459 K |
| log P | 2.721 |
| Vapor pressure | 30 Pa (at 20 °C) |
Refractive index (nD)
|
1.431 |
| Thermochemistry | |
Heat capacity (C)
|
317.5J K−1 mol−1 |
Std molar
entropy (S |
347.0 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−433.67–−432.09 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
−5.28857–−5.28699 MJ mol−1 |
| Hazards | |
| Main hazards | Mildly toxic |
| GHS pictograms |  
|
| GHS Signal word | DANGER |
| H302, H312, H315, H318, H335 | |
| P261, P280, P305+351+338 | |
| Flash point | 81 °C (178 °F; 354 K) |
| 290 °C (554 °F; 563 K) | |
| Explosive limits | 0.88–9.7% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
none[2] |
REL (Recommended)
|
TWA 50 ppm (270 mg/m3) [skin][2] |
IDLH (Immediate danger)
|
N.D.[2] |
| Related compounds | |
Related alkanol
|
Propylheptyl alcohol |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
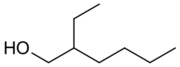
2-Ethylhexanol (abbreviated 2-EH) is an organic compound with formula C8H18O. It is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a large scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers.[3] It is encountered in plants, fruits, and wines.[4][5] The odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.[6][7]
Properties and applications
The branching in 2-ethylhexanol inhibits crystallization. Esters of 2-ethylhexanol are similarly affected, which together with low volatility, is the basis of applications in the production of plasticizers and lubricants, where its presence helps reduce viscosity and lower freezing points. Because 2-ethylhexanol is a fatty alcohol, its esters have emollient properties. Representative is the diester bis(2-ethylhexyl) phthalate (DEHP), commonly used in PVC. The triester tris (2-Ethylhexyl) trimellitate (TOTM) is another common plasticizer produced via the esterification of three 2-ethylhexanol per trimellitic acid.
It is also commonly used as a low volatility solvent. 2-Ethylhexanol can also be used as a cetane improver when reacted with nitric acid. It also used to react with epichlorohydrin and sodium hydroxide to produce 2-Ethylhexyl glycidyl ether which is then used as an epoxy reactive diluent in various coatings, adhesives and sealants applications. It can be used in the development of photos, production of rubber and extraction of oil and gas.[8]
Industrial production
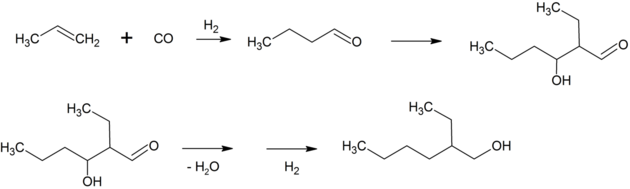
2-Ethylhexanol is produced industrially by the aldol condensation of n-butyraldehyde, followed by hydrogenation of the resulting hydroxyaldehyde. About 2,500,000 tons are prepared in this way annually.[9][10]
The n-butyraldehyde is made by hydroformylation of propylene, either in a self-contained plant or as the first step in a fully integrated facility. Most facilities make n-butanol and isobutanol in addition to 2-ethylhexanol. Alcohols prepared in this way are sometimes referred to as oxo alcohols. The overall process is very similar to that of the Guerbet reaction, by which it may also be produced.[11]
Health effects
2-Ethylhexanol exhibits low toxicity in animal models, with LD50 ranging from 2-3 g/kg (rat).[3] 2-Ethylhexanol has been identified as a cause of indoor air quality related health problems, such as respiratory system irritation, as a volatile organic compound. 2-Ethylhexanol is emitted to air from a PVC flooring installed on concrete that had not been dried properly.[12][13]
2-Ethylhexanol has been linked to developmental toxicity (increased incidence of skeletal malformations in fetuses).[14] This is thought to be a result of metabolism of 2-ethylhexanol into 2-ethylhexanoic acid via oxidation of the primary alcohol.[15][16] The teratogenicity of 2-ethylhexanoic acid, as well as similar substances such as valproic acid, has been well established.[17][18][19][20][21]
Nomenclature
Although isooctanol (and the derived isooctyl prefix) is commonly used in industry to refer to 2-ethylhexanol and its derivatives, IUPAC naming conventions[22] dictate that this name is properly applied to another isomer of octanol, 6-methylheptan-1-ol. The Chemical Abstracts Service likewise indexes isooctanol (CAS# 26952-21-6) as 6-methylheptan-1-ol.
See also
References
- ↑ "2-ethylhexanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=7720.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0354". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0354.html.
- ↑ 3.0 3.1 Helmut Bahrmann; Heinz-Dieter Hahn; Dieter Mayer (2005). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_137. ISBN 978-3-527-30673-2.
- ↑ Fan, Wenlai; Qian, Michael C. (2006). "Characterization of Aroma Compounds of Chinese "Wuliangye" and "Jiannanchun" Liquors by Aroma Extract Dilution Analysis". Journal of Agricultural and Food Chemistry 54 (7): 2695–2704. doi:10.1021/jf052635t. PMID 16569063.
- ↑ Mayuoni-Kirshinbaum, Lina; Tietel, Zipora; Porat, Ron; Ulrich, Detlef (2012). "Identification of aroma-active compounds in 'wonderful' pomegranate fruit using solvent-assisted flavour evaporation and headspace solid-phase micro-extraction methods". European Food Research and Technology 235 (2): 277–283. doi:10.1007/s00217-012-1757-0.
- ↑ Klaus Rettinger; Christian Burschka; Peter Scheeben; Heike Fuchs; Armin Mosandl (1991). "Chiral 2-alkylbranched acids, esters and alcohols. Preparation and stereospecific flavour evaluation". Tetrahedron: Asymmetry 2 (10): 965–968. doi:10.1016/S0957-4166(00)86137-6. https://opus.bibliothek.uni-wuerzburg.de/files/4494/Burschka_Esters.pdf.
- ↑ McGinty, D.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. (2010). "Fragrance material review on 2-ethyl-1-hexanol". Food and Chemical Toxicology 48: S115–S129. doi:10.1016/j.fct.2010.05.042. PMID 20659633.
- ↑ "Product Spotlight: 2-Ethylhexanol". December 12, 2019. https://berrymanchemical.com/blog/2-ethylhexanol/.
- ↑ C. Kohlpaintner; M. Schulte; J. Falbe; P. Lappe; J. Weber (2008). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_321.pub2. ISBN 978-3-527-30673-2.
- ↑ Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 4180-4181.
- ↑ Miller, Robert; Bennett, George (January 1961). "Producing 2-Ethylhexanol by the Guerbet Reaction". Industrial & Engineering Chemistry 53 (1): 33–36. doi:10.1021/ie50613a027.
- ↑ Ernstgård, L.; Norbäck, D. (2010). "Acute effects of exposure to 1 mg/m(3) of vaporized 2-ethyl-1-hexanol in humans". Indoor Air 20 (2): 168–75. doi:10.1111/j.1600-0668.2009.00638.x. PMID 20409194.
- ↑ Hildenbrand, S.; Wodarz, R. (2009). "Biomonitoring of the di(2-ethylhexyl) phthalate metabolites mono(2-ethyl-5-hydroxyhexyl) phthalate and mono(2-ethyl-5-oxohexyl) phthalate in children and adults during the course of time and seasons.". International Journal of Hygiene and Environmental Health 212 (6): 679–84. doi:10.1016/j.ijheh.2009.06.003. PMID 19615938.
- ↑ "2-Ethylhexanol: Provisional Peer-Reviewed Toxicity Values (PPRTVs)" (in en). US EPA: National Center for Environmental Assessment. https://cfpub.epa.gov/ncea/pprtv/chemicalLanding.cfm?pprtv_sub_id=2005.
- ↑ "Metabolism of 2-ethylhexanol administered orally and dermally to the female Fischer-344 rat" (in en). 2009-03-15. https://hero.epa.gov/index.cfm/reference/details/reference_id/3046161.
- ↑ Eastman Kodak Company, Eastman Kodak (2009-03-15). "Pharmacokinetic studies with 2-ethylhexanol in the female fischer 344 rat (final report) with attachments and cover letter dated 050791" (in en). https://hero.epa.gov/index.cfm/reference/details/reference_id/3006683.
- ↑ Pennanen, Sirpa; Tuovinen, Kai; Huuskonen, Hannele; Komulainen, Hannu (1992). "The developmental toxicity of 2-ethylhexanoic acid in Wistar rats". Fundamental and Applied Toxicology 19 (4): 505–511. doi:10.1016/0272-0590(92)90088-Y. PMID 1426708. https://www.sciencedirect.com/science/article/abs/pii/027205909290088Y.
- ↑ "2-Ethylhexanoic acid". ACGIH. https://haz-map.com/Agents/1405.
- ↑ "Annex 1 Background Document to the Opinion proposing harmonised classification and labelling at EU level of 2-Ethylhexanoic acid and its salts, with the exception of those specified elsewhere in this Annex". European Chemicals Agency. 2020. https://echa.europa.eu/documents/10162/c58bf900-5d67-463f-3d45-1e09009a1e22.
- ↑ "Valproic Acid". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/valproic-acid.html.
- ↑ "Valproate banned without the pregnancy prevention programme". https://www.gov.uk/government/news/valproate-banned-without-the-pregnancy-prevention-programme.
- ↑ "Rule A-2. Saturated Branched-chain Compounds and Univalent Radicals (ACYCLIC HYDROCARBONS)". https://www.acdlabs.com/iupac/nomenclature/79/r79_36.htm.
External links
- Isooctyl alcohol, National Institute for Occupational Safety and Health (NIOSH)
 |