Chemistry:2-Hexyne
From HandWiki
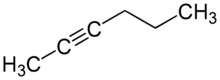
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hex-2-yne | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10 | |
| Molar mass | 82.146 g·mol−1 |
| Density | 0.7317 |
| Melting point | −88 °C (−126 °F; 185 K)[1] |
| Boiling point | 83.8 °C (182.8 °F; 356.9 K)[2] |
Refractive index (nD)
|
1.4135 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H225, H304, H315, H319 | |
| P210, P233, P240, P241, P242, P243, P264, P280, P301+310, P302+352, P303+361+353, P305+351+338, P321, P331, P332+313, P337+313, P362, P370+378, P403+235, P405, P501 | |
| Related compounds | |
Related compounds
|
3-Hexyne, 1-Hexyne |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Hexyne is an organic compound that belongs to the alkyne group. Just like its isomers, it also has the chemical formula of C6H10.
Reactions
2-Hexyne can be semihydrogenated to yield 2-hexene or fully hydrogenated to hexane.[3] With appropriate noble metal catalysts it can selectively form cis-2-hexene.[4]
2-Hexyne can act as a ligand on gold atoms.[5]
With strong sulfuric acid, the ketone 2-hexanone is produced. However this reaction also causes polymerization and charring.[6]
Under heat and pressure 2-hexyne polymerizes to linear oligomers and polymers. This can be hastened by some catalysts such as molybdenum pentachloride with tetraphenyl tin. However Ziegler–Natta catalysts have no action as the triple bond is hindered.[7]
References
- ↑ Campbell, Kenneth N.; Eby, Lawrence T. (October 1941). "The Reduction of Multiple Carbon—Carbon Bonds. III. Further Studies on the Preparation of Olefins from Acetylenes 1,2". Journal of the American Chemical Society 63 (10): 2683–2685. doi:10.1021/ja01855a050.
- ↑ Hennion, G. F.; Sheehan, J. J. (June 1949). "1,2-Hexadiene". Journal of the American Chemical Society 71 (6): 1964–1966. doi:10.1021/ja01174a017.
- ↑ Ulan, Judith G.; Maier, Wilhelm F. (September 1989). "Mechanism of 2-hexyne hydrogenation on heterogeneous palladium". Journal of Molecular Catalysis 54 (2): 243–261. doi:10.1016/0304-5102(89)80220-2.
- ↑ Schrock, Richard R.; Osborn, John A. (April 1976). "Catalytic hydrogenation using cationic rhodium complexes. II. The selective hydrogenation of alkynes to cis olefins". Journal of the American Chemical Society 98 (8): 2143–2147. doi:10.1021/ja00424a021.
- ↑ Zuccaccia, Daniele; Belpassi, Leonardo; Rocchigiani, Luca; Tarantelli, Francesco; Macchioni, Alceo (5 April 2010). "A Phosphine Gold(I) π-Alkyne Complex: Tuning the Metal−Alkyne Bond Character and Counterion Position by the Choice of the Ancillary Ligand". Inorganic Chemistry 49 (7): 3080–3082. doi:10.1021/ic100093n. PMID 20222666.
- ↑ Thomas, Robert J.; Campbell, Kenneth N.; Hennion, G. F. (March 1938). "Catalytic Hydration of Alkylacetylenes 1". Journal of the American Chemical Society 60 (3): 718–720. doi:10.1021/ja01270a061.
- ↑ Higashimura, Toshinobu; Deng, Yun Xiang; Masuda, Toshio (March 1982). "Polymerization of 2-hexyne and higher 2-alkynes catalyzed by MoCl 5 Ph 4 Sn and WCl 6 Ph 4 Sn 1". Macromolecules 15 (2): 234–238. doi:10.1021/ma00230a005.
 |
