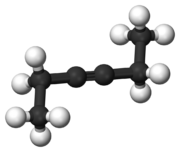
Chemistry:3-Hexyne
From HandWiki
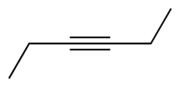
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hex-3-yne | |
| Other names
Diethylacetylene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10 | |
| Molar mass | 82.14 g/mol |
| Appearance | Colorless liquid |
| Density | 0.723 g/cm3 |
| Melting point | −105 °C (−157 °F; 168 K) |
| Boiling point | 81 to 82 °C (178 to 180 °F; 354 to 355 K) |
| low | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H225, H304, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+310, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P331, P332+313, P337+313, P362, P370+378, P403+233, P403+235, P405 | |
| Flash point | −14 °C (7 °F; 259 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Hexyne is the organic compound with the formula C2H5CCC2H5. This colorless liquid is one of three isomeric hexynes. 3-Hexyne forms with 5-decyne, 4-octyne, and 2-butyne a series of symmetric alkynes. It is a reagent in organometallic chemistry.[1]
thumb|left|144px|Structure of the [[coordination complex NbCl3(dimethoxyethane)(3-hexyne).[2]]]
References
- ↑ Maynard, R. B.; Borodinsky, L.; Grimes, R. N. (1984). "2,3-diethyl-2,3-dicarba- nido -hexaborane(8)". Inorganic Syntheses. 22. pp. 211–214. doi:10.1002/9780470132531.ch49. ISBN 9780470132531.
- ↑ Arteaga-Müller, Rocío; Tsurugi, Hayato; Saito, Teruhiko; Yanagawa, Masao; Oda, Seiji; Mashima, Kazushi (2009). "New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism". Journal of the American Chemical Society 131 (15): 5370–5371. doi:10.1021/ja8100837. PMID 20560633.
 |
