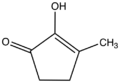
Chemistry:2-Hydroxy-3-methyl-2-cyclopenten-1-one
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Methylcyclopentane-1,2-dione | |
| Other names
Maple lactone, Methylcyclopentenolone, Corylon
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O2 | |
| Molar mass | 112.128 g·mol−1 |
| Density | 1.312 g/cm3 |
| Melting point | 104–108 °C (219–226 °F; 377–381 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Hydroxy-3-methyl-2-cyclopenten-1-one is an organic compound related to 1,2-cyclopentanedione. It is the enol tautomer of the diketone 3-methylcyclopentane-1,2-dione. Being an enol, the compound is often called methylcyclopentenolone. It is a colorless solid.
Synthesis and structure
The compound is prepared by base-catalyzed condensation of 1‐hydroxyhexane‐2,5‐dione, a derivative of hydroxymethylfurfural.[2]
The structure has been confirmed by X-ray crystallography.[2] Quantum calculations also indicate that the enol is strongly favored relative to the diketo tautomer. Furthermore, the enolization occurs at the methyl-substituted carbon.[3]
Use and occurrence
It is one of many products from the pyrolysis of lignocellulose.[4]
It is used in flavors and perfumery for its maple- or caramel-like odor.[5] It contributes to the flavor or odor of many foods including wines, coffee, paprika, and salmon. It is sometimes called maple lactone because it occurs in maple syrup (it is not, however, a lactone).[6]
See also
References
- ↑ 3-Methyl-1,2-cyclopentanedione at Sigma Aldrich
- ↑ 2.0 2.1 Wozniak, Bartosz; Spannenberg, Anke; Li, Yuehui; Hinze, Sandra; De Vries, Johannes G. (2018). "Cyclopentanone Derivatives from 5-Hydroxymethylfurfural via 1-Hydroxyhexane-2,5-dione as Intermediate". ChemSusChem 11 (2): 356–359. doi:10.1002/cssc.201702100. PMID 29235723.
- ↑ Zborowski, Krzysztof K.; Grybos, Ryszard; Wesełucha-Birczyńska, Aleksandra; Kim, Younkyoo; Proniewicz, Leonard M. (2012). "Quantum Mechanical Study of the Tautomerism and Molecular Spectra of 2-Hydroxy-3-methyl-2-cyclopenten-1-one". Molecular Physics 110 (6): 343–351. doi:10.1080/00268976.2011.646336. Bibcode: 2012MolPh.110..343Z.
- ↑ Zhang, Huiyan; Cheng, Yu-Ting; Vispute, Tushar P.; Xiao, Rui; Huber, George W. (2011). "Catalytic Conversion of Biomass-Derived Feedstocks into Olefins and Aromatics with ZSM-5: The Hydrogen to Carbon Effective Ratio". Energy & Environmental Science 4 (6): 2297. doi:10.1039/C1EE01230D. https://works.bepress.com/cgi/viewcontent.cgi?article=1022&context=george_huber.
- ↑ "Cyclotene". Good Scents Company. http://www.thegoodscentscompany.com/data/rw1012711.html.
- ↑ Ball, David W. (2007). "The Chemical Composition of Maple Syrup". Journal of Chemical Education 84 (10): 1647. doi:10.1021/ed084p1647. Bibcode: 2007JChEd..84.1647B.
 |

