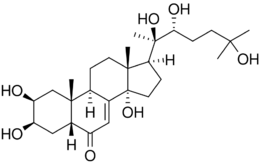
Chemistry:20-Hydroxyecdysone
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 4-9 hours |
| Excretion | Urinary:?% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C27H44O7 |
| Molar mass | 480.642 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
20-Hydroxyecdysone (ecdysterone or 20E) is a naturally occurring ecdysteroid hormone which controls the ecdysis (moulting) and metamorphosis of arthropods. It is therefore one of the most common moulting hormones in insects, crabs, etc. It is also a phytoecdysteroid produced by various plants, including Cyanotis vaga, Ajuga turkestanica and Rhaponticum carthamoides where its purpose is presumably to disrupt the development and reproduction of insect pests. In arthropods, 20-hydroxyecdysone acts through the ecdysone receptor. Although mammals lack this receptor, 20-hydroxyecdysone affects mammalian biological systems. 20-Hydroxyecdysone is an ingredient of some supplements that aim to enhance physical performance. In humans, it is hypothesized to bind to the estrogen receptor beta (ERβ) protein-coding gene.[1]
Sources in arthropods
The primary sources of 20-hydroxyecdysone in larvae are the prothoracic gland, ring gland, gut, and fat bodies. These tissues convert dietary cholesterol into the mature forms of the hormone 20-hydroxyecdysone.[2] For the most part these glandular tissues are lost in the adult with exception of the fat body, which is retained as a sheath of lipid tissue surrounding the brain and organs of the abdomen. In the adult female the ovary is a substantial source of 20-hydroxyecdysone production.[3] Adult males are left with, so far as is currently known, one source of 20-hydroxyecdysone which is the fat body tissue. These hormone producing tissues express the ecdysone receptor throughout development, possibly indicating a functional feedback mechanism.
Ecdysteroid activity in arthropods
An ecdysteroid is a type of steroid hormones in insects that are derived from enzymatic modification of cholesterol by p450 enzymes. This occurs by a mechanism similar to steroid synthesis in vertebrates. Ecdysone and 20-hydroxyecdysone regulate larval molts, onset of puparium formation, and metamorphosis. Being that these hormones are hydrophobic, they traverse lipid membranes and permeate the tissues of an organism. Indeed, the main receptor of these hormone signals - the ecdysone receptor - is an intracellular protein.
In humans and other mammals
Use as supplement
20-Hydroxyecdysone and other ecdysteroids are marketed as ingredients in nutritional supplements for various sports, particularly bodybuilding.[4] A 2006 study looked for improvement in actual exercises performed and tested for improvements/increases in chemical indicators such as body composition and free/available testosterone and concluded that using 30 mg per day of 20-hydroxyecdysone administered orally did not significantly affect anabolic or catabolic responses to resistance training, body composition, or training adaptations.[5] However, a number of earlier studies supported the anabolic effects of 20-Hydroxyecdysone.[6][7][8][9][10][11] A more recent study conducted in 2019 by a team that included the Department for Molecular and Cellular Sports Medicine at the German Sport University Cologne, found that significantly higher increases in muscle mass were observed in participants dosed with ecdysterone, with significantly more pronounced increases in one-repetition bench press performance.[12] The study was funded by the World Anti-Doping Agency (WADA) and demonstrated a significant dose-responsive anabolic effect of 20-Hydroxyecdysone supplementation on athletes during resistance training. Furthermore, recent studies have elucidated that the mechanism of action of 20-Hydroxyecdysone on human muscle cells is relatively selective activation of the beta form of the estrogen receptor (ERβ),[13] which is known to result in muscle hypertrophy.[14][promotional source?]
Use as research tool
20-Hydroxyecdysone and other ecdysteroids are used in biochemistry research as inducers in transgenic animals, whereby a new gene is introduced into an animal so that its expression is under the control of an introduced ecdysone receptor. Adding or removing ecdysteroids from the animal's diet then gives a convenient way to turn the inserted gene on or off (see ecdysone receptor). At usual doses, 20-hydroxyecdysone appears to have little or no effect on animals that do not have extra genes inserted; it also has high bioavailability when taken orally, so it is useful for determining whether the transgene has been taken up effectively.[15]
External links
- Ecdybase, The Ecdysone Handbook - a free online ecdysteroids database
References
- ↑ "Ecdysteroids as non-conventional anabolic agent: performance enhancement by ecdysterone supplementation in humans". Archives of Toxicology 93 (7): 1807–1816. July 2019. doi:10.1007/s00204-019-02490-x. PMID 31123801.
- ↑ "Steroid signaling in plants and insects--common themes, different pathways". Genes & Development 16 (24): 3113–29. December 2002. doi:10.1101/gad.1042102. PMID 12502734. http://www.genesdev.org/cgi/reprint/16/24/3113.
- ↑ "Ecdysteroid titers during pupal and adult development in Drosophila melanogaster". Developmental Biology 93 (1): 73–82. September 1982. doi:10.1016/0012-1606(82)90240-8. PMID 6813165.
- ↑ "Analysis of Ingredients of Supplements in the National Institutes of Health Supplement Database Marketed as Containing a Novel Alternative to Anabolic Steroids". JAMA Network Open 3 (4): e202818. April 2020. doi:10.1001/jamanetworkopen.2020.2818. PMID 32293681.
- ↑ "Effects of methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males". Journal of the International Society of Sports Nutrition 3 (2): 19–27. December 2006. doi:10.1186/1550-2783-3-2-19. PMID 18500969.
- ↑ "The Combined Use of Ecdisten and the Product'Bodrost'during Training in Cyclical Types of Sport.". Scientific Sports Bulletin: 2. 1988.
- ↑ "[A comparative study of the anabolic action of ecdysten, leveton and Prime Plus, preparations of plant origin.]". Eksperimental'naia i Klinicheskaia Farmakologiia 58 (5): 46–8. 1995. PMID 8704590.
- ↑ "Phytoecdysteroids and anabolic-androgenic steroids--structure and effects on humans". Current Medicinal Chemistry 15 (1): 75–91. 2008. doi:10.2174/092986708783330674. PMID 18220764. http://publicatio.bibl.u-szeged.hu/10883/1/2008_Bathori_et_al._CMC_u.pdf.
- ↑ The influence of preparations of plant origin on physical work capacity. (Report). The Russian Ministry of Public Health. 1986.
- ↑ Comments on the Results of Retibol in the Practice of Athletic Training and Rehabilitation (Report). Natural Sports Research Institute.
- ↑ "[The effect of the antioxidants elton and leveton on the physical work capacity of athletes]" (in ru). Eksperimental'naia i Klinicheskaia Farmakologiia 61 (1): 60–2. 1998. PMID 9575416.
- ↑ "Ecdysteroids as non-conventional anabolic agent: performance enhancement by ecdysterone supplementation in humans". Archives of Toxicology 93 (7): 1807–1816. July 2019. doi:10.1007/s00204-019-02490-x. PMID 31123801.
- ↑ "Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone". Molecular Nutrition & Food Research 58 (9): 1861–72. September 2014. doi:10.1002/mnfr.201300806. PMID 24974955.
- ↑ "Ecdysterone: Definition, Benefits, & Side Effects". https://turkesterone.com/pages/ecdysterone.
- ↑ "Identification of ligands and coligands for the ecdysone-regulated gene switch". Proceedings of the National Academy of Sciences of the United States of America 97 (26): 14512–7. December 2000. doi:10.1073/pnas.260499497. PMID 11114195. PMC 18950. Bibcode: 2000PNAS...9714512S. http://www.pnas.org/cgi/reprint/97/26/14512.pdf.
 |
