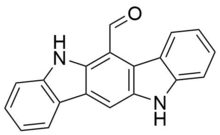
Chemistry:6-formyl-indolo(3,2-b)carbazole
| |
| Names | |
|---|---|
| IUPAC name
5,11-Dihydroindolo[3,2-b]carbazole-6-carboxaldehyde
| |
| Other names
6-Formylindolo[3,2-b]carbazole
FICZ | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H12N2O | |
| Molar mass | 284.318 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
6-formyl-indolo[3,2-b]carbazole (FICZ) (chemical formula C19H12N2O) is a nitrogen heterocycle, having an extremely high affinity (Kd = 7 x 10-11M)[1][2] for binding to the Aryl hydrocarbon receptor (AhR).
It was originally identified as a photooxidized derivative of the amino acid tryptophan and suggested to be the endogenous liagand of the AhR.[1]
Occurrence
FICZ can be found in any solution, including cell culture media, containing the amino acid tryptophan, especially if exposed to Ultraviolet light or visible light.[3][4][5] In human keratinocyte (HaCaT) cells treated with Trp and thereafter irradiated with UVB formation of intracellular FICZ could also be demonstrated. [6] In a similar way FICZ has also been identified and quantified in Jurkat cells incubated in tL-Trp enriched medium.[7] FICZ was first identified in humans as sulfogonjugates Sulfation, a type of metabolites of FICZ, by use of liquid chromatography (LC) coupled with mass spectrometry (MS) (LC/MS/MS) Liquid chromatography–mass spectrometry.[5] FICZ has been identified in the skin of persons with the disease vitiligo Vitiligo.[8] and in extracts of skin originating from patients with the Malassezia-associated diseases Seborrhoeic dermatitis (SD) or Pityriasis versicolor (PV).[9] Malassezia yeasts are commensal microorganisms found on the skin on many animals including humans. When the yeast stain Malassezia furfur is cultured on agar containing Trp as the only nitrogen source it produces a variety of indole derivatives some of which activate AhR e.g. FICZ. [10][11][12] The gastrointestinal tract is a rich source of microorganisms and a favorable environment for formation of indoles and indole derivatives. [13] Although FICZ itself has not yet been identified in mouse cecum extract or fecal pellets other indole derivatives that are precursors of FICZ e.g. indole-3-pyruvate and tryptamine have been found [14].
Biosynthesis and mechanisms of formation
Microbiota both on human skin and in the gut can convert Trp to several metabolites, some of which with an affinity for the AHR.[15] In the first report on the structure elucidation of the Trp photo-oxidation product FICZ indole-3-acetaldehyde (I3A) was suggested to be a precursor of FICZ [2]
In addition to the light or H2O2-induced formation of FICZ, a number of other enzymatic pathways have been identified to convert Trp to FICZ via the precursor indole-3-acetaldehyde (I3A).[12] The oxidation product of I3A is indole-3 acetic acid, the most common natural plant hormone auxin Auxin. Decarboxylation of Trp catalyzed by the enzyme aromatic L-amino acid decarboxylase (AADC) yields tryptamine, while decarboxylation of 5-hydroxy-Trp, by the same enzyme also (called DOPA decarboxylase) gives serotonin.Serotonin Tryptamine is then oxidatively deaminated by monoamine oxidases MAO-A and B) Monoamine oxidase to produce the FICZ precursor I3A. Post incubation after deamination of the reaction mixture in the absence of an active enzyme generate FICZ but also its oxidation product CICZ. These non-enzymatic reactions were favored by low pH or increased temperatur.[12]

Chemical syntheses
The indolo[3,2-b]carbazoles [16] have been intensely studied as synthetic targets due to their diverse biological effects numerous applications in materials chemistry. The double Fischer indolization for the synthesis of the parent system indolo[3,2-b]carbazole was first reported by Robinson 1963 [17]and has since been applied for the synthesis of FICZ and related structures. [16] A more practical chemical synthesis of FICZ has been reported by Zhang et al. (2019)[18] using readily available and commercially obtainable starting materials such as 1-(phenylsulfonyl)-1H-indole and 1-(phenylsulfonyl)-1H-indole-3-carbaldehyde. In order to reach gram amounts in the multistep synthesis of this low solubility ring-closed carbazole (FICZ) the final purification by column chromatography was replaced by a crystallization step instead.
AHR-binding and gene induction
When the high-affinity AHR ligand FICZ binds to the receptor, which is a ligand dependent transcription factor activation of many target genes takes place. The most well-studied of these target genes is the cytochrome P450 (CYP) 1A1 Cytochrome P450. The CYPS are a superfamily of enzymes involved in the metabolism of large number of both endogenous and exogenous compounds. The first chemical compound to be recognized as a high affinity AHR ligand was 2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD. The CYP-induction by TCDD is sustained while the induction by FICZ is transient[19] because of its rapid metabolic degradation by CYP1A1. [20] Various effects of the AHR and FICZ in cancer cells and cancer stem cells (CSCs) have also been described. Moreover, it is generally believed that sustained AHR activation can be prooncogenic, dysregulating key physiological processes and thereby influencing tumor initiation, promotion, and progression, as well as metastasis in a cell-type specific manner.[21]
Metabolism
In a first analysis of the metabolism of FICZ S9 fractions prepared from mouse Hepa-1 cells [22] were used. Three HPLC fractions representing FICZ metabolites were occurring with time in the treated S9 derived from the wild type cells while no metabolites were detected in the CYP1A1-deficient cells.[23] In the further analyses of the CYP1A1 derived metabolites two monohydroxylated FICZ metabolites (2-hydroxyindolo[3,2-b]carbazole-6-carboxaldehyde and 8-hydroxyindolo[3,2-b]carbazole-6-carboxaldehyde) and three dihydroxylated metabolites 2,8-, 2,10-, and 4,8-dihydroxyindolo[3,2-b]carbazole-6-carboxaldehyde respectively, were identified by LC-MS and NMR spectroscopy Nuclear magnetic resonance spectroscopyand the chemical structure of the metabolites were confirmed by chemical analyses.[24] Kinetic studies using human recombinant CYP1A1, -1A2, and -1B1 expressed in E. coli showed that the catalytic efficiencies (kcat/Km) Specificity constant were found to be 5–50-fold higher for FICZ than for the standard substrates 7-ethoxyresorufin and 7-metoxyrsorufin and at least 5000-fold higher compared with the hydroxylation of estrogens.[5] CYP1A1 catalyzed hydroxylation of FICZ extremely efficiently, with kcat/Km of 8.1x107 M-1 s-1. The catalytic efficiency for FICZ as a CYP1A1 substrate is close to the limit of diffusion, thus FICZ is an excellent CYP1A1 substrate, but is also a very good substrate for CYP1A2 and CYP1B1.[5] The mono- and di-hydroxylated metabolites of FICZ are subject to further metabolic changes to glucuronide- and sulfate-conjugates.[25] Sulfotransferase (SULT) catalyzed reactions resulted in a more pronounced reduction of the hydroxylated FICZ metabolites than the glucuronidation. Further studies using human recombinant SULTs showed that SULT1A1, -1A2, -1B1 and -1E1 exhibited high catalytic efficiencies and 2-hydroxylated FICZ was more efficiently conjugated than the 8-hydroxylated FICZ. With a kcat/Km of 1.1 107 M-1 s-1 with 2-OH-FICZ as substrate SULT1A2 exhibited a higher values than for any other substrate. Also dihydroxylated FICZ metabolites are converted to disulfuric acid esters but at a slower rate with intermediate formation of monosulfoconjugates, one of which was identified in human urine.[5]
The FICZ/AHR/CYP1A1 feedback loop
Already in the 1980s Nebert and coworkers proposed that a feedback loop involving an endogenous AHR ligand that is also a substrate for CYP1A1regulates this signaling.[22] FICZ also has the capacity to inhibit CYP1A1 activity, but the inhibition is transient since FICZ is such an exceptionally good substrate for the CYP1A1 enzyme.[19] There are several substances, both exogenous and endogenous, which can inhibit CYP1A1 leading to an accumulation of FICZ in the cell and a subsequent activation of the AHR and induction of CYP1A1.[26][21]This feedback system is essential for the physiological function of the AHR signaling, since the AHR regulates the balance between the quiescence and proliferation of a large number of cells, such as intra-thymic progenitor cells, as well as hematopoietic, pulmonary, and neuro-epithelial stem cells.[21]
Physiological functions mediated by FICZ
Self-renewal and differentiation of stem/progenitor cells
The AHR seems to play important roles in normal embryonic development and a reversible repression of the receptor is essential for the maintenance of the pluripotency of embryonic stem cells (ESC).[27] It has been shown that expansion of early progenitor murine hematopoietic stem cells is promoted by down-regulation of AHR signaling through the RNA-binding protein Musashi-2 and 250 nM FICZ reversed this effect.[28]Furthermore, expansion of human induced pluripotent stem cells was enhanced by the AHR inhibitor CH223191 and blocked by FICZ.[29]In contrast, by applying a novel, pluripotent stem-cell based in vitro culture system Smith et al. demonstrated that the potent AhR ligand FICZ resulted in an exponential expansion (600-fold increase) of induced pluripotent stem cells (iPSC)-derived hematopoetic progenitor cell (HP) populations. Furthermore, FICZ treatment for extended periods of time (60 days) resulted in a progressive erythroid specification and maturation of the HP cells.[30] In addition, ex vivo treatment of rat hepatic progenitor cells with 1-100nM FICZ led to both sustained AHR activation and triggering of cell proliferation.[31]
Various effects of the AHR and FICZ in cancer cells and cancer stem cells (CSCs) have also been described. Moreover, it is generally believed that sustained AHR activation can be prooncogenic, dysregulating key physiological processes and thereby influencing tumor initiation, promotion, and progression, as well as metastasis in a cell-type specific manner.[32]Treatment of breast cancer cells with 500nM FICZ, for instance, has been shown to increase aldehyde dehydrogenase 1 (ALDH1) a marker for CSCs in adult cancers and 100nM FICZ treatment of head and neck squamous carcinoma cell lines did up-regulate several growth factors. On the other hand, did FICZ cause cell cycle and proliferation arrest in colon cancer cells, as well as inhibition of tumor growth in mice implanted with T-cell lymphoma or B16 melanoma cells. [21]Stockinger and coworkers demonstrated the importance of CYP1A1 for the endogenous availability of FICZ. Th17 cells from mice expressing CYP1A1 constitutively metabolized FICZ rapidly and responded with lower production of IL-22 when treated with 0.01nM FICZ compared to wildtype Th17 cells. Metabolic clearance of FICZ in mice that overexpress Cyp1a1 in the gut epithelium led to a pseudo-AHR-deficient state and when infected with Citrobacter rodentium, these animals exhibited markedly reduced numbers of group 3 ILCs and Th17 cells and succumbed rapidly.
Immune responses and differences in immune cells
The AHR is involved in the regulation of T helper 17 cell (Th17) and regulatory T cell (Treg) differentiation, which is of importance for the treatment of autoimmunity, infections and cancer. The initial set of experiments indicated a ligand specificity in the response, i.e. AHR activation by TCDD induced functional Treg cells, while activation by FICZ boosted Th17 cell differentiation. In later studies, however, no such effects were found and it was confirmed that only the dose and duration of AHR activation by the high-affinity ligands are the primary drivers of T cell differentiation. AHR activation by FICZ can promote the development of Th17 cells causing inflammation and autoimmunity, but also promote an expansion of the Treg cell population and thereby stimulate immunosuppressive activity.Taking rapid metabolic degradation of FICZ into account, there seems to be no intrinsic difference in the effects of FICZ and TCDD on T cell differentiation and T cell-mediated adaptive immune responses. Similarly, FICZ can stimulate or inhibit cytokine production and the maturation and homeostasis of Mastcells in vitro, as well as anaphylactic responses in vivo, depending on the dose and timing of exposure.[21]
Effects of FICZ on immune barriers
The AHR is highly expressed in cells of the immune barrier organs, such as skin, lung, gut, and mucosal epithelia, as well as in the placenta.[33] The intestinal epithelium is a relatively impermeable physical and immunological barrier. Intestinal epithelial cells participate in the inflammatory and immune responses when activated by interleukin 22 (IL-22). Innate lymphoid cells seem to be one dominant endogenous sours of IL-22.[34]AHR-deficient mice have fewer intestinal IELs and ILCs and therefore a reduced expression of IL-22 and do not survive an infection by the intestinal pathogen Citrobacter rodentium. It has been found that in wildtype mice, FICZ increased the production of IL-22 by the ILCs.[35]Daily i.p. injections of 100 mg kg-1 FICZ to adult mice dramatically reduced their mortality following infection with the intestinal pathogen Listeria monocytogenes.[36]Stockinger and coworkers demonstrated the importance of CYP1A1 for the endogenous availability of FICZ. Th17 cells from mice expressing CYP1A1 constitutively metabolized FICZ rapidly and responded with lower production of IL-22 when treated with 0.01nM FICZ compared to wildtype Th17 cells. Metabolic clearance of FICZ in mice that overexpress Cyp1a1 in the gut epithelium led to a pseudo-AHR-deficient state and when infected with Citrobacter rodentium, these animals exhibited markedly reduced numbers of group 3 ILCs and Th17 cells and succumbed rapidly. Conversely, mice lacking CYP1A1 or when CYP1A1 is inhibited results in an elevate level of endogenous FICZ and conversely, mice lacking CYP1A1 or with inhibited CYP1A1 activity show elevated levels of endogenous FICZ and increased protection against intestinal infection.[37] [38] The composition of the murine commensal microbiota influences susceptibility to gastrointestinal infections and induced colitis and specific components of this microbiota promote the production of AHR ligands resulting in protection against intestinal damage induced by dextran sulfate sodium (DSS). Lamas and co-workers have shown that mice with dysbiotic microbiota due to their lack of the caspase recruitment domain 9 (CARD9) produced lower levels of endogenous AHR agonists and recovered more poorly from DSS-induced colitis. When 1 µg FICZ was injected i.p. one day after DSS administration, the severity of colitis in these animals was reduced significantly. Also, the effects of defects in the colonic expression of IL-22 and genes coding for antimicrobial proteins in Card9 KO mice could be reversed by FICZ. Administration of AhR agonists in different animal models of colitis has underscored the role of AhR in gut inflammation. After FICZ treatment, the severity of different types of induced colitis in mice significantly decreased, characterized by the down-regulation of pro-inflammatory cytokines and production of IL-22. Moreover, administration of anti-IL-22 prevented the anti-inflammatory effect of FICZ, demonstrating that the therapeutic effect of FICZ at least is partially mediated by IL- 22.[39][40] The effect of AhR ligands in reversing inflammatory responses has also been demonstrated in a clinical setting. FICZ treatment of lamina propria mononuclear cells from Crohn’s disease patients resulted in decreased IFN-γ expression and up-regulation of IL-22.[41]
FICZ is also involved in the physiological regulation of Th2-mediated immunity in the lung. FICZ markedly inhibited the lipopolysaccharide- and ovalbumin-induced proliferation of T cells. FICZ likewise suppresses pulmonary Th2-type cytokine production in a mouse model of ovalbumin-induced allergic asthma.
The key roles played by dynamic AHR signaling in skin immunity have been emphasized by Stockinger and coworkers (Di Meglio et al. 2014). When full-thickness biopsies from the lesional skin of patients with psoriasis was exposed to FICZ, 29 genes belonging to the psoriasis transcriptome were down-regulated.[42] A later murine study confirmed that FICZ decreased IL-17 expression and lessened the severity of psoriasis. Similarly, FICZ and AHR may be involved in the etiology of cutaneous systemic lupus erythematosus and atopic dermatitis. There are also evidence for an important physiological role for FICZ in the expression of IL-22 in the skin. The uptake of Trp and intracellular accumulation of FICZ in skin γδ T cells is regulated by the activation marker CD69 in combination with the aromatic-amino-acid-transporter complex LAT1-CD98. These results revealed the importance of Trp uptake for AHR dependent secretion of IL-22 by γδ T cells during the development of psoriasis.[43]
The toxicity of FICZ
Several findings indicate that high levels of FICZ can exert ROS-dependent toxicity, whereas low doses can transiently elevate local levels ROS/Ca2+, thereby promoting cellular adaptation, survival, and proliferation. FICZ has proven to be potently embryotoxic toward fish and birds. Zebrafish embryos demonstrated a dramatically increased mortality and severe toxicity, however only when CYP1A1 was inhibited.[44]FICZ can also act as a nanomolar photosensitizer potentiating UVA-induced oxidative stress irrespective of AHR ligand.[45]
References
- ↑ 1.0 1.1 Rannug, A; Rannug, U; Rosenkranz, HS; Winqvist, L; Westerholm, R; Agurell, E; Grafström, AK (15 November 1987). "Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances.". The Journal of biological chemistry 262 (32): 15422-7. PMID 2824460.
- ↑ 2.0 2.1 Rannug, U; Rannug, A; Sjöberg, U; Li, H; Westerholm, R; Bergman, J (December 1995). "Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands.". Chemistry & biology 2 (12): 841-5. PMID 8807817.
- ↑ Oberg, M; Bergander, L; Håkansson, H; Rannug, U; Rannug, A (June 2005). "Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity.". Toxicological sciences : an official journal of the Society of Toxicology 85 (2): 935-43. doi:10.1093/toxsci/kfi154. PMID 15788723.
- ↑ Diani-Moore, S; Labitzke, E; Brown, R; Garvin, A; Wong, L; Rifkind, AB (March 2006). "Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo.". Toxicological sciences : an official journal of the Society of Toxicology 90 (1): 96-110. doi:10.1093/toxsci/kfj065. PMID 16330490.
- ↑ 5.0 5.1 5.2 5.3 5.4 Wincent, E; Amini, N; Luecke, S; Glatt, H; Bergman, J; Crescenzi, C; Rannug, A; Rannug, U (30 January 2009). "The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans.". The Journal of biological chemistry 284 (5): 2690-6. doi:10.1074/jbc.M808321200. PMID 19054769.
- ↑ Fritsche, E; Schäfer, C; Calles, C; Bernsmann, T; Bernshausen, T; Wurm, M; Hübenthal, U; Cline, JE et al. (22 May 2007). "Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation.". Proceedings of the National Academy of Sciences of the United States of America 104 (21): 8851-6. doi:10.1073/pnas.0701764104. PMID 17502624.
- ↑ Cibrián, Danay; Sánchez-Madrid, Francisco (June 2017). "CD69: from activation marker to metabolic gatekeeper". European Journal of Immunology 47 (6): 946–953. doi:10.1002/eji.201646837. ISSN 1521-4141. PMID 28475283. PMC 6485631. https://www.ncbi.nlm.nih.gov/pubmed/28475283.
- ↑ Schallreuter, KU; Salem, MA; Gibbons, NC; Maitland, DJ; Marsch, E; Elwary, SM; Healey, AR (June 2012). "Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: Epidermal H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling.". FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26 (6): 2471-85. doi:10.1096/fj.11-201897. PMID 22415306.
- ↑ Magiatis, Prokopios; Pappas, Periklis; Gaitanis, George; Mexia, Nikitia; Melliou, Eleni; Galanou, Maria; Vlachos, Christophoros; Stathopoulou, Konstantina et al. (August 2013). "Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin". The Journal of Investigative Dermatology 133 (8): 2023–2030. doi:10.1038/jid.2013.92. ISSN 1523-1747. PMID 23448877. PMC 3714356. https://www.ncbi.nlm.nih.gov/pubmed/23448877.
- ↑ Gaitanis, G; Magiatis, P; Hantschke, M; Bassukas, ID; Velegraki, A (January 2012). "The Malassezia genus in skin and systemic diseases.". Clinical microbiology reviews 25 (1): 106-41. doi:10.1128/CMR.00021-11. PMID 22232373.
- ↑ Magiatis, P; Pappas, P; Gaitanis, G; Mexia, N; Melliou, E; Galanou, M; Vlachos, C; Stathopoulou, K et al. (August 2013). "Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin.". The Journal of investigative dermatology 133 (8): 2023-30. doi:10.1038/jid.2013.92. PMID 23448877.
- ↑ 12.0 12.1 12.2 Smirnova, A; Wincent, E; Vikström Bergander, L; Alsberg, T; Bergman, J; Rannug, A; Rannug, U (19 January 2016). "Evidence for New Light-Independent Pathways for Generation of the Endogenous Aryl Hydrocarbon Receptor Agonist FICZ.". Chemical research in toxicology 29 (1): 75-86. doi:10.1021/acs.chemrestox.5b00416. PMID 26686552.
- ↑ Bansal, T; Alaniz, RC; Wood, TK; Jayaraman, A (5 January 2010). "The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation.". Proceedings of the National Academy of Sciences of the United States of America 107 (1): 228-33. doi:10.1073/pnas.0906112107. PMID 19966295.
- ↑ Zelante, T; Iannitti, RG; Cunha, C; De Luca, A; Giovannini, G; Pieraccini, G; Zecchi, R; D'Angelo, C et al. (22 August 2013). "Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22.". Immunity 39 (2): 372-85. doi:10.1016/j.immuni.2013.08.003. PMID 23973224.
- ↑ Murray, Iain A.; Perdew, Gary H. (February 2017). "Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis". Current Opinion in Toxicology 2: 15–23. doi:10.1016/j.cotox.2017.01.003. ISSN 2468-2934. PMID 28944314. PMC 5604257. https://www.ncbi.nlm.nih.gov/pubmed/28944314.
- ↑ 16.0 16.1 Janosik, Tomasz; Rannug, Agneta; Rannug, Ulf; Wahlström, Niklas; Slätt, Johnny; Bergman, Jan (26 September 2018). "Chemistry and Properties of Indolocarbazoles". Chemical Reviews 118 (18): 9058–9128. doi:10.1021/acs.chemrev.8b00186. ISSN 1520-6890. PMID 30191712. https://www.ncbi.nlm.nih.gov/pubmed/30191712.
- ↑ Robinson, B. (1963). "568. The Fischer indolisation of cyclohexane-1,4-dione bisphenylhydrazone" (in en). Journal of the Chemical Society (Resumed): 3097. doi:10.1039/jr9630003097. ISSN 0368-1769. http://xlink.rsc.org/?DOI=jr9630003097.
- ↑ Zhang, Cunyu; Creech, Katrina L.; Zuercher, William J.; Willson, Timothy M. (10 July 2019). "Gram-scale synthesis of FICZ, a photoreactive endogenous ligand of the aryl hydrocarbon receptor" (in en). Scientific Reports 9 (1): 1–9. doi:10.1038/s41598-019-46374-7. ISSN 2045-2322. PMID 31292477. PMC 6620467. https://www.nature.com/articles/s41598-019-46374-7.
- ↑ 19.0 19.1 Wei, Y. D.; Helleberg, H.; Rannug, U.; Rannug, A. (12 March 1998). "Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-bcarbazole"]. Chemico-Biological Interactions 110 (1-2): 39–55. doi:10.1016/s0009-2797(97)00111-7. ISSN 0009-2797. PMID 9566724. https://www.ncbi.nlm.nih.gov/pubmed/9566724.
- ↑ Wei, Y. D.; Bergander, L.; Rannug, U.; Rannug, A. (2000). "Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-bcarbazole"]. Archives of Biochemistry and Biophysics 383 (1): 99–107. doi:10.1006/abbi.2000.2037. ISSN 0003-9861. PMID 11097181. https://www.ncbi.nlm.nih.gov/pubmed/11097181.
- ↑ 21.0 21.1 21.2 21.3 21.4 Rannug, Agneta; Rannug, Ulf (August 2018). "The tryptophan derivative 6-formylindolo[3,2-bcarbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation"]. Critical Reviews in Toxicology 48 (7): 555–574. doi:10.1080/10408444.2018.1493086. ISSN 1547-6898. PMID 30226107. https://www.ncbi.nlm.nih.gov/pubmed/30226107.
- ↑ 22.0 22.1 Hankinson, O; Andersen, RD; Birren, BW; Sander, F; Negishi, M; Nebert, DW (10 February 1985). "Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line.". The Journal of biological chemistry 260 (3): 1790-5. PMID 3968086.
- ↑ Wei, YD; Bergander, L; Rannug, U; Rannug, A (1 November 2000). "Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole.". Archives of biochemistry and biophysics 383 (1): 99-107. doi:10.1006/abbi.2000.2037. PMID 11097181.
- ↑ Bergander, L; Wahlström, N; Alsberg, T; Bergman, J; Rannug, A; Rannug, U (February 2003). "Characterization of in vitro metabolites of the aryl hydrocarbon receptor ligand 6-formylindolo[3,2-b]carbazole by liquid chromatography-mass spectrometry and NMR.". Drug metabolism and disposition: the biological fate of chemicals 31 (2): 233-41. PMID 12527705.
- ↑ Bergander, Linda; Wincent, Emma; Rannug, Agneta; Foroozesh, Maryam; Alworth, William; Rannug, Ulf (2004). "Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-bcarbazole"]. Chemico-Biological Interactions 149 (2-3): 151–164. doi:10.1016/j.cbi.2004.08.005. ISSN 0009-2797. PMID 15501436. https://www.ncbi.nlm.nih.gov/pubmed/15501436.
- ↑ Wincent, Emma; Bengtsson, Johanna; Mohammadi Bardbori, Afshin; Alsberg, Tomas; Luecke, Sandra; Rannug, Ulf; Rannug, Agneta (2012). "Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor". Proceedings of the National Academy of Sciences of the United States of America 109 (12): 4479–4484. doi:10.1073/pnas.1118467109. ISSN 1091-6490. PMID 22392998. PMC 3311358. https://www.ncbi.nlm.nih.gov/pubmed/22392998.
- ↑ Ko, Chia-I.; Wang, Qin; Fan, Yunxia; Xia, Ying; Puga, Alvaro (January 2014). "Pluripotency factors and Polycomb Group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells". Stem Cell Research 12 (1): 296–308. doi:10.1016/j.scr.2013.11.007. ISSN 1876-7753. PMID 24316986. PMC 3896086. https://www.ncbi.nlm.nih.gov/pubmed/24316986.
- ↑ Rentas, Stefan; Holzapfel, Nicholas; Belew, Muluken S; Pratt, Gabriel; Voisin, Veronique; Wilhelm, Brian T; Bader, Gary D; Yeo, Gene W et al. (2016-04-28). "Musashi-2 Attenuates AHR Signaling to Expand Human Hematopoietic Stem Cells". Nature 532 (7600): 508–511. doi:10.1038/nature17665. ISSN 0028-0836. PMID 27121842. PMC 4880456. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880456/.
- ↑ Leung, Amy; Zulick, Elizabeth; Skvir, Nicholas; Vanuytsel, Kim; Morrison, Tasha A.; Naing, Zaw Htut; Wang, Zhongyan; Dai, Yan et al. (July 2018). "Notch and Aryl Hydrocarbon Receptor Signaling Impact Definitive Hematopoiesis from Human Pluripotent Stem Cells". Stem Cells (Dayton, Ohio) 36 (7): 1004–1019. doi:10.1002/stem.2822. ISSN 1066-5099. PMID 29569827. PMC 6099224. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6099224/.
- ↑ Smith, Brenden W.; Rozelle, Sarah S.; Leung, Amy; Ubellacker, Jessalyn; Parks, Ashley; Nah, Shirley K.; French, Deborah; Gadue, Paul et al. (2013-07-18). "The aryl hydrocarbon receptor directs hematopoietic progenitor cell expansion and differentiation". Blood 122 (3): 376–385. doi:10.1182/blood-2012-11-466722. ISSN 1528-0020. PMID 23723449. PMC 3716202. https://www.ncbi.nlm.nih.gov/pubmed/23723449.
- ↑ Harrill, Joshua A.; Parks, Bethany B.; Wauthier, Eliane; Rowlands, J. Craig; Reid, Lola M.; Thomas, Russell S. (February 2015). "Lineage-dependent effects of aryl hydrocarbon receptor agonists contribute to liver tumorigenesis". Hepatology (Baltimore, Md.) 61 (2): 548–560. doi:10.1002/hep.27547. ISSN 1527-3350. PMID 25284723. PMC 4303521. https://www.ncbi.nlm.nih.gov/pubmed/25284723.
- ↑ Wang, Zhongyan; Monti, Stefano; Sherr, David H. (2017-02-01). "The diverse and important contributions of the AHR to cancer and cancer immunity" (in en). Current Opinion in Toxicology. Mechanistic Toxicology 2: 93–102. doi:10.1016/j.cotox.2017.01.008. ISSN 2468-2020. http://www.sciencedirect.com/science/article/pii/S2468202016300365.
- ↑ Esser, Charlotte; Rannug, Agneta (April 2015). Ma, Qiang. ed. "The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology" (in en). Pharmacological Reviews 67 (2): 259–279. doi:10.1124/pr.114.009001. ISSN 0031-6997. http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pr.114.009001.
- ↑ Gf, Sonnenberg; La, Fouser; D, Artis (May 2011). "Border Patrol: Regulation of Immunity, Inflammation and Tissue Homeostasis at Barrier Surfaces by IL-22" (in en). https://pubmed.ncbi.nlm.nih.gov/21502992/.
- ↑ J, Qiu; Jj, Heller; X, Guo; Zm, Chen; K, Fish; Yx, Fu; L, Zhou (27 January). "The Aryl Hydrocarbon Receptor Regulates Gut Immunity Through Modulation of Innate Lymphoid Cells" (in en). doi:10.1016/j.immuni.2011.11.011. https://pubmed.ncbi.nlm.nih.gov/22177117/.
- ↑ A, Kimura; H, Abe; S, Tsuruta; S, Chiba; Y, Fujii-Kuriyama; T, Sekiya; R, Morita; A, Yoshimura (April 2014). "Aryl Hydrocarbon Receptor Protects Against Bacterial Infection by Promoting Macrophage Survival and Reactive Oxygen Species Production" (in en). https://pubmed.ncbi.nlm.nih.gov/24343818/.
- ↑ Schiering, Chris; Wincent, Emma; Metidji, Amina; Iseppon, Andrea; Li, Ying; Potocnik, Alexandre J.; Omenetti, Sara; Henderson, Colin J. et al. (2017). "Feedback control of AHR signalling regulates intestinal immunity". Nature 542 (7640): 242–245. doi:10.1038/nature21080. ISSN 1476-4687. PMID 28146477. PMC 5302159. https://www.ncbi.nlm.nih.gov/pubmed/28146477.
- ↑ Schiering, Chris; Vonk, Anne; Das, Srustidhar; Stockinger, Brigitta; Wincent, Emma (May 2018). "Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells". Biochemical Pharmacology 151: 47–58. doi:10.1016/j.bcp.2018.02.031. ISSN 1873-2968. PMID 29501585. https://www.ncbi.nlm.nih.gov/pubmed/29501585.
- ↑ Lamas, Bruno; Richard, Mathias L.; Leducq, Valentin; Pham, Hang-Phuong; Michel, Marie-Laure; Da Costa, Gregory; Bridonneau, Chantal; Jegou, Sarah et al. (June 2016). "CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands". Nature Medicine 22 (6): 598–605. doi:10.1038/nm.4102. ISSN 1546-170X. PMID 27158904. PMC 5087285. https://www.ncbi.nlm.nih.gov/pubmed/27158904.
- ↑ Lamas, Bruno; Natividad, Jane M.; Sokol, Harry (July 2018). "Aryl hydrocarbon receptor and intestinal immunity". Mucosal Immunology 11 (4): 1024–1038. doi:10.1038/s41385-018-0019-2. ISSN 1935-3456. PMID 29626198. https://www.ncbi.nlm.nih.gov/pubmed/29626198.
- ↑ Monteleone, Ivan; Rizzo, Angelamaria; Sarra, Massimiliano; Sica, Giuseppe; Sileri, Pierpaolo; Biancone, Livia; MacDonald, Thomas T.; Pallone, Francesco et al. (July 2011). "Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract". Gastroenterology 141 (1): 237–248, 248.e1. doi:10.1053/j.gastro.2011.04.007. ISSN 1528-0012. PMID 21600206. https://www.ncbi.nlm.nih.gov/pubmed/21600206.
- ↑ Di Meglio, Paola; Duarte, João H.; Ahlfors, Helena; Owens, Nick D. L.; Li, Ying; Villanova, Federica; Tosi, Isabella; Hirota, Keiji et al. (19 June 2014). "Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions". Immunity 40 (6): 989–1001. doi:10.1016/j.immuni.2014.04.019. ISSN 1097-4180. PMID 24909886. PMC 4067745. https://www.ncbi.nlm.nih.gov/pubmed/24909886.
- ↑ Cibrian, Danay; Saiz, María Laura; de la Fuente, Hortensia; Sánchez-Díaz, Raquel; Moreno-Gonzalo, Olga; Jorge, Inmaculada; Ferrarini, Alessia; Vázquez, Jesús et al. (August 2016). "CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis". Nature Immunology 17 (8): 985–996. doi:10.1038/ni.3504. ISSN 1529-2916. PMID 27376471. PMC 5146640. https://www.ncbi.nlm.nih.gov/pubmed/27376471.
- ↑ Wincent, Emma; Kubota, Akira; Timme-Laragy, Alicia; Jönsson, Maria E.; Hahn, Mark E.; Stegeman, John J. (2016). "Biological effects of 6-formylindolo[3,2-bcarbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner"]. Biochemical Pharmacology 110-111: 117–129. doi:10.1016/j.bcp.2016.04.012. ISSN 1873-2968. PMID 27112072. PMC 4887394. https://www.ncbi.nlm.nih.gov/pubmed/27112072.
- ↑ Park, Sophia L.; Justiniano, Rebecca; Williams, Joshua D.; Cabello, Christopher M.; Qiao, Shuxi; Wondrak, Georg T. (June 2015). "The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo[3,2-bCarbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes"]. The Journal of Investigative Dermatology 135 (6): 1649–1658. doi:10.1038/jid.2014.503. ISSN 1523-1747. PMID 25431849. PMC 4430374. https://www.ncbi.nlm.nih.gov/pubmed/25431849.
