Chemistry:Alkanolamine
In organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl (–OH) and amino (–NH
2, –NHR, and –NR
2) functional groups on an alkane backbone. Most alkanolamines are colorless.[1]
- Alkanolamines
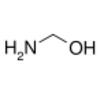
methanolamine, from the reaction of ammonia with formaldehyde
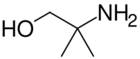
2-amino-2-methyl-1-propanol is a precursor to oxazolines
Sphingosine is a component of some cell membrane.
1-Aminoalcohols
1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member.
2-Aminoalcohols
2-Aminoalcohols are an important class of organic compounds that are often generated by the reaction of amines with epoxides:
- C
2H
4O + R–NH
2 → RNHC
2H
4OH
Simple alkanolamines are used as solvents, synthetic intermediates, and high-boiling bases.[2]
Hydrogenation or hydride reduction of amino acids gives the corresponding 2-aminoalcohols. Examples include prolinol (from proline), valinol (from valine), tyrosinol (from tyrosine).
Key members: ethanolamine, dimethylethanolamine, N-methylethanolamine, Aminomethyl propanol. Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinally useful derivative of ethanolamine.[citation needed]
1,3-, 1,4-, and 1,5-amino alcohols
- Heptaminol, a cardiac stimulant
- Propanolamines
Natural products
Most proteins and peptides contain both alcohols and amino groups. Two amino acids are alkanolamines, formally speaking: serine and hydroxyproline.
- Veratridine and veratrine
- Tropane alkaloids such as atropine
- hormones and neurotransmitters epinephrine (adrenaline) and norepinephrine (noradrenaline)
References
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1, https://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ↑ Martin Ernst; Johann-Peter Melder; Franz Ingo Berger; Christian Koch (2022). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001.pub2.
External links
- Amino+Alcohols at the US National Library of Medicine Medical Subject Headings (MeSH)
 |