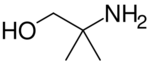
Chemistry:Aminomethyl propanol
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Amino-2-methylpropan-1-ol | |
| Other names
Isobutanol-2-amine; Aminoisobutanol; 2-Amino-2-methyl-1-propanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H11NO | |
| Molar mass | 89.138 g·mol−1 |
| Density | 0.934 g/cm3 |
| Melting point | 30–31 °C (86–88 °F; 303–304 K) |
| Boiling point | 165.5 °C (329.9 °F; 438.6 K) |
| Miscible | |
| Solubility in alcohols | Soluble |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aminomethyl propanol is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.[1]
Synthesis
Aminomethyl propanol can be produced by the hydrogenation of 2-aminoisobutyric acid or its esters.
Properties
Aminomethyl propanol is soluble in water[2][3] and about the same density as water.[2]
Uses
Aminomethyl propanol is used for the preparation of buffer solutions.[2] It is a component of the drugs ambuphylline and pamabrom. It is also used in cosmetics.[1]
It is a precursor to oxazolines via its reaction with acyl chlorides.[4] Via sulfation of the alcohol, the compound is also a precursor to 2,2-dimethylaziridine.[5]
It is used in the synthesis of Fepradinol & G-130. It is also used for Isobucaine, and Radafaxine.
References
- ↑ 1.0 1.1 "Aminomethyl-propanol". http://cosmeticsinfo.org/ingredient/aminomethyl-propanol.
- ↑ 2.0 2.1 2.2 "2-Amino-2-methyl-1-propanol". http://www.chemicalbook.com/ChemicalProductProperty_EN_CB0320043.htm.
- ↑ Bougie, Francis; Iliuta, Maria (2012-02-14). "Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams". J Chem Eng Data 57 (3): 635–669. doi:10.1021/je200731v.
- ↑ Albert I. Meyers; Mark E. Flanagan (1993). "2,2'-Dimethoxy-6-Formylbiphenyl". Org. Synth. 71: 107. doi:10.15227/orgsyn.071.0107.
- ↑ Kenneth N. Campbell; Armiger H. Sommers; Barbara K. Campbell; Lee Irvin Smith; Oliver H. Emerson; D. E. Pearson; J. F. Baxter; K. N. Carter (1947). "Tert-butylamine". Org. Synth. 27: 12. doi:10.15227/orgsyn.027.0012.
 |


