Chemistry:Ammonium bromide
| |
| |
| Names | |
|---|---|
| IUPAC name
Ammonium bromide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
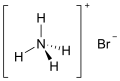
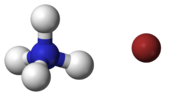
| NH4Br | |
| Molar mass | 97.94 g/mol |
| Appearance | white powder, hygroscopic |
| Density | 2.429 g/cm3 |
| Melting point | 235 °C (455 °F; 508 K) |
| Boiling point | 452 °C (846 °F; 725 K) |
| 60.6 g/100 mL (0 °C) 78.3 g/100 mL (25 °C) 145 g/100 mL (100 °C) | |
| −47.0×10−6 cm3/mol | |
Refractive index (nD)
|
1.712 |
| Structure | |
| Isometric | |
| Hazards | |
| GHS pictograms |  [1] [1]
|
| GHS Signal word | Warning |
| H315, H319, H335[1] | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Ammonium fluoride Ammonium chloride Ammonium iodide |
Other cations
|
Sodium bromide Potassium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ammonium bromide, NH4Br, is the ammonium salt of hydrobromic acid. The chemical crystallizes in colorless prisms, possessing a saline taste; it sublimes on heating and is easily soluble in water. On exposure to air it gradually assumes a yellow color because of the oxidation of traces of bromide (Br−) to bromine (Br2).
Preparation
Ammonium bromide can be prepared by the direct action of hydrogen bromide on ammonia.
- NH3 + HBr → NH4Br
It can also be prepared by the reaction of ammonia with iron(II) bromide or iron(III) bromide, which may be obtained by passing aqueous bromine solution over iron filings.
- 2 NH3 + FeBr2 + 2 H2O → 2 NH4Br + Fe(OH)2
Reactions
Ammonium bromide is a weak acid with a pKa of approximately 5 in water. It is an acid salt because the ammonium ion hydrolyzes slightly in water.
Ammonium bromide is a strong electrolyte when put in water:
- NH4Br(s) → NH+
4(aq) + Br−(aq)
Ammonium bromide decomposes to ammonia and hydrogen bromide when heated at elevated temperatures:
- NH4Br → NH3 + HBr
Uses
Ammonium bromide is used for photography in films, plates and papers; in fireproofing of wood; in lithography and process engraving; in corrosion inhibitors; and in pharmaceutical preparations.[2]
References
- ↑ 1.0 1.1 Sigma-Aldrich Co., Ammonium bromide.
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN:0-07-049439-8
 |


