Chemistry:Ammonium metavanadate
| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium trioxovanadate(V)
| |
| Other names
Ammonium vanadate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| NH4VO3 | |
| Molar mass | 116.98 g/mol |
| Appearance | white |
| Density | 2.326 g/cm3 |
| Melting point | >200 °C (392 °F; 473 K)[1] (decomposes) |
| 4.8 g/100 ml (20 °C)[1] | |
| Solubility | soluble in diethanolamine, ethanolamine |
| Hazards | |
| Main hazards | possible mutagen, dangerous for the environment |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H301, H332, H340, H361, H370, H372, H412 | |
| P201, P202, P260, P261, P264, P270, P271, P273, P281, P301+310, P304+312, P304+340, P307+311, P308+313, P312, P314, P321, P330, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
58.1 mg/kg, oral (rat) |
| Related compounds | |
Other anions
|
Ammonium orthovanadate Ammonium hexavanadate |
Other cations
|
Sodium metavanadate Potassium metavanadate |
Related compounds
|
Vanadium pentoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
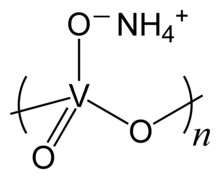
Ammonium metavanadate is the inorganic compound with the formula NH4VO3. It is a white salt, although samples are often yellow owing to impurities of V2O5. It is an important intermediate in the purification of vanadium.[2]
Synthesis and structure
The compound is prepared by the addition of ammonium salts to solutions of vanadate ions, generated by dissolution of V2O5 in basic aqueous solutions, such as hot sodium carbonate. The compound precipitates as a colourless solid.[3][4] This precipitation step can be slow.
The compound adopts a polymeric structure consisting of chains of [VO3]−, formed as corner-sharing VO4 tetrahedra. These chains are interconnected via hydrogen bonds with ammonium ions.[5]
 |
150px | 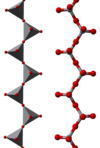
|
| ball-and-stick model | polyhedral model | [(VO3)n]n− chains |
Uses
Vanadium is often purified from aqueous extracts of slags and ore by selective precipitation of ammonium metavanadate. The material is then roasted to give vanadium pentoxide:[2]
- 2 NH4VO3 → V2O5 + 2 NH3 + H2O
Other
Vanadates can behave as structural mimics of phosphates, and in this way they exhibit biological activity.[6][7]
Ammonium metavanadate is used to prepare Mandelin reagent, a qualitative test for alkaloids.[citation needed]
References
- ↑ 1.0 1.1 John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–40. ISBN 978-1138561632.
- ↑ 2.0 2.1 Günter Bauer, Volker Güther, Hans Hess, Andreas Otto, Oskar Roidl, Heinz Roller, Siegfried Sattelberger "Vanadium and Vanadium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a27_367
- ↑ G. Brauer "Ammonium Metavanadate" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1272.
- ↑ Robert H. Baker, Harry Zimmerman, R. N. Maxson "Ammonium Metavanadate" Inorganic Syntheses, 1950, Vol. 3, 117-118. doi:10.1002/9780470132340.ch30
- ↑ Vladimír Syneček and František Hanic (1954). "The crystal structure of ammonium metavanadate". Czechoslovak Journal of Physics 4 (2): 120–129. doi:10.1007/BF01687750. Bibcode: 1954CzJPh...4..120S.
- ↑ Korbecki, Jan; Baranowska-Bosiacka, Irena; Gutowska, Izabela; Chlubek, Dariusz "Biochemical and medical importance of vanadium compounds" Acta Biochimica Polonica 2012, vol. 59, pp. 195-200.
- ↑ Crans, D. C.; Chatterjee, P. B. "Vanadium Biochemistry" Reedijk, Jan; Poeppelmeier, Kenneth, Eds. Comprehensive Inorganic Chemistry II (2013), 3, 323-342. doi:10.1016/B978-0-08-097774-4.00324-7
 |


