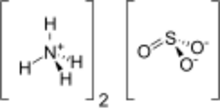
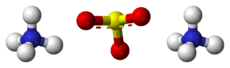
Chemistry:Ammonium sulfite
| |
| |
| Names | |
|---|---|
| IUPAC name
Ammonium sulfite
| |
| Other names
Ammonium sulphite, Diammonium sulfite, Diammonium sulfonate, Sulfurous acid, Diammonium salt, Sulfurous acid, ammonium salt(1:2)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3077 |
| |
| |
| Properties | |
| (NH4)2SO3 | |
| Molar mass | 116.14 g/mol |
| Appearance | colourless[1] hygroscopic crystals[2] |
| Melting point | 65 °C (149 °F; 338 K) decomposes[1] |
| 35 g/100 mL[1]
32.4g/100mL at 0 degrees Celsius[3] 60.4g/100mL at 100 degrees Celsius[3] | |
| Solubility | Insoluble in acetone and alcohol[2] |
Refractive index (nD)
|
1.515.[3] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P321, P332+313, P337+313, P362, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Not Flammable[2] |
| Related compounds | |
Other anions
|
Ammonium hydroxide Ammonium thiosulfate Ammonium sulfate Ammonium bisulfate Ammonium persulfate |
Other cations
|
Sodium sulfite Potassium sulfite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3.
Preparation
Ammonium sulfite can be prepared by the reaction of ammonia with sulfur dioxide in aqueous solution:
- 2 NH3 + SO2 + H2O → (NH4)2SO3
Ammonium sulfite is produced in gas scrubbers, now obsolete, consisting of ammonium hydroxide to remove sulfur dioxide from emissions from power plants. The conversion is the basis of the Walther Process. The resulting ammonium sulfite can be air oxidized to give ammonium sulfate.[4]
Uses
Ammonium sulfite is the precursor to ammonium thiosulfate, by reaction with elemental sulfur.
Niche
For cosmetics, ammonium sulfite is used as a hair straightening agent and a hair waving agent.[5] Ammonium based hair products have been made to replace sodium hydroxide-based products due to the destructive nature of sodium hydroxide on hair.
The most common food product with ammonium sulfite is caramel coloring E150d. According to the FDA, caramel coloring contains ammonium, potassium, or sodium sulfite.[6]
Ammonium sulfite is used as a preservative for fixers in photography. When film photographs are being developed ammonium sulfite can be one of the reducing agents used to preserve the hypo (sodium thiosulfate or ammonium thiosulfate).[7]
Ammonium sulfite can also be used in the making of bricks. The bricks made using ammonium sulfite are mainly used for blast furnace linings.[8]
Ammonium sulfite can be included in lubricants for cold metal working. The lubricants are intended to reduce friction to keep heat production down and keep impurities out of the metals.[8]
Chemical properties
Ammonium sulfite is a reducing agent.[9] It emits sulfur dioxide and oxides of nitrogen upon heating to decomposition.
The specific gravity of ammonium sulfite is 1.41.[2] The refractive index of ammonium sulfite is 1.515.[3]
References
- ↑ 1.0 1.1 1.2 "Chemical Entity Data Page". http://www.chemthes.com/entity_datapage.php?id=3512.
- ↑ 2.0 2.1 2.2 2.3 "Material Safety Data Sheet: Ammonium sulfite MSDS. accessed Oct 19, 2011)". http://www.sciencelab.com/msds.php?msdsId=9922932.
- ↑ 3.0 3.1 3.2 3.3 Pubchem. "SID 167823 - PubChem". https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?sid=167823.
- ↑ Karl-Heinz Zapp (2012). "Ammonium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_243. ISBN 978-3527306732.
- ↑ Europe. European Commission. Health and Consumers. Cosmetics - CosIng [Cosmetics Directive (v.1)]. European Commission. Web. 26 Oct. 2011.
- ↑ United States. FDA. CFR - Code of Federal Regulations Title 21. Health and Human Services. 1 Apr. 2011. Web
- ↑ Haist, Grant Milford (1979). Modern photographic processing.. New York: Wiley. ISBN 0-471-02228-4. OCLC 251467968. https://www.worldcat.org/oclc/251467968.
- ↑ 8.0 8.1 O'Neil, Maryadele J. The Merck Index: an Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck, 2001. 561. Print.
- ↑ "Ammoniumsulfit - 10196-04-0". http://www.chemicalbook.com/ChemicalProductProperty_DE_CB0309297.htm.
 |


