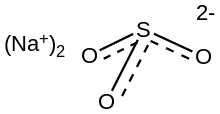
Chemistry:Sodium sulfite
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Sodium sulfite
| |||
| Other names
Hypo clear (photography)
E221 | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| Na2SO3 | |||
| Molar mass | 126.043 g/mol | ||
| Appearance | White solid | ||
| Odor | Odorless | ||
| Density | 2.633 g/cm3 (anhydrous) 1.561 g/cm3 (heptahydrate) | ||
| Melting point | 33.4 °C (92.1 °F; 306.5 K) (dehydration of heptahydrate) 500 °C (anhydrous) | ||
| Boiling point | Decomposes | ||
| 27.0 g/100 mL water (20 °C) | |||
| Solubility | Soluble in glycerol Insoluble in ammonia, chlorine | ||
| log P | −4 | ||
| Acidity (pKa) | ~9 (heptahydrate) | ||
Refractive index (nD)
|
1.565 | ||
| Structure | |||
| Hexagonal (anhydrous) Monoclinic (heptahydrate) | |||
| Hazards | |||
| Safety data sheet | ICSC 1200 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Sodium selenite | ||
Other cations
|
Potassium sulfite | ||
Related compounds
|
Sodium bisulfite Sodium metabisulfite Sodium sulfate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. It is also suitable for the softening of lignin in the pulping and refining processes of wood and lignocellulosic materials.[1] A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.[2]
Preparation
Structure of anhydrous sodium sulfite|left|thumb. Sodium sulfite can be prepared by treating a solution of sodium hydroxide with sulfur dioxide. When conducted in warm water, Na2SO3 initially precipitates as a white solid. With more SO2, the solid dissolves to give the disulfite, which crystallizes upon cooling.[2]
Sodium sulfite is made industrially by treating sulfur dioxide with a solution of sodium carbonate.[3] The overall reaction is:
Uses
Sodium sulfite is primarily used in the pulp and paper industry.[4] It has been also applied in the thermomechanical conversion of wood to fibres (defibration) for producing medium density fibreboards (MDF).[5]
As an oxygen scavenger agent, it is used to treat water being fed to steam boilers to avoid corrosion problems,[6] in the photographic industry, it protects developer solutions from oxidation and (as hypo clear solution) to wash fixer (sodium thiosulfate) from film and photo-paper emulsions.
As a reducing agent it is used in the textile industry as a bleaching, desulfurizing, and dechlorinating agent (e.g. in swimming pools). Its reducing properties are exploited in its use as a preservative to prevent dried fruit from discoloring, and for preserving meats.
It is used as a reagent in sulfonation and sulfomethylation agent. It is used in the production of sodium thiosulfate.
The Wellman–Lord process utilizes sodium sulfite for flue gas desulfurization.
Reactions
Sodium sulfite is primarily used as a mild reducing agent. The heptahydrate crystals effloresce in warm dry air. Heptahydrate crystals also oxidize in air to form sodium sulfate. The anhydrous form is more resistant to oxidation by air.[7]
Sodium bisulfite, NaHSO3, is mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions.
Structure
According to X-ray crystallography sodium sulfite heptahydrate features pyramidal SO32- centers. The S-O distances are 1.50 and the O-S-O angles are near 106º.[8]
References
- ↑ "High Yield Pulp Production by Modified Sulfite Process". https://ippta.co/wp-content/uploads/2021/01/IPPTA-VII1-39-42-High-Yield-Pulp-Production.pdf.
- ↑ 2.0 2.1 Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. 2. pp. 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- ↑ Weil, Edward D.; Sandler, Stanley R. (1999). "Sulfur Compounds". in Kroschwitz, Jacqueline I.. Kirk-Othmer Concise Encyclopedia of Chemical Technology (4th ed.). New York: John Wiley & Sons, Inc.. p. 1937. ISBN 978-0471419617.
- ↑ Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- ↑ "DE19958756A1 - Production of light-colored medium-density fibreboard (MDF) from old fibreboard comprises treatment with sodium sulfite, conversion into pulp and feeding into the blow-line of an MDF plant". 1999-12-07. https://patents.google.com/patent/DE19958756A1/en.
- ↑ "Pre-boiler and Boiler Corrosion Control | GE Water". http://www.gewater.com/handbook/boiler_water_systems/ch_11_preboiler.jsp.
- ↑ Merck Index of Chemicals and Drugs, 9th ed. monograph 8451
- ↑ Larsson, Lars Olof; Kierkegaard, Peder; Lindberg, Bengt; Holme, Tord; Lindberg, Alf A.; Craig, J. Cymerman (1969). "The Crystal Structure of Sodium Sulphite. A Refinement Allowing for the Effect of Crystal Twinning". Acta Chemica Scandinavica 23: 2253–2260. doi:10.3891/acta.chem.scand.23-2253.
 |



