Chemistry:Bemotrizinol
| |
| Names | |
|---|---|
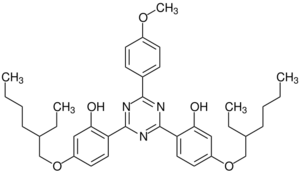
| Preferred IUPAC name
2,2′-[6-(4-Methoxyphenyl)-1,3,5-triazine-2,4-diyl]bis{5-[(2-ethylhexyl)oxy]phenol} | |
| Other names
Tinosorb S
Bis-ethylhexyloxyphenol methoxyphenyl triazine Anisotriazine | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | BEMT |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C38H49N3O5 | |
| Molar mass | 627.826 g·mol−1 |
| Hazards | |
| H413 | |
| P273, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bemotrizinol (INN[1][2]/USAN,[3] INCI bis-ethylhexyloxyphenol methoxyphenyl triazine) is an oil-soluble organic compound that is added to sunscreens to absorb UV rays. It is marketed as Parsol Shield, Tinosorb S, and Escalol S.
Bemotrizinol is a broad-spectrum UV absorber, absorbing UVB as well as UVA rays. It has two absorption peaks, 310 and 340 nm.[4] It is highly photostable. Even after 50 MEDs (minimal erythemal doses), 98.4% remains intact. It helps prevent the photodegradation of other sunscreen actives like avobenzone.[5] A recent development is Tinosorb S Aqua, which is bemotrizinol in a PMMA matrix dispersed in water. This makes it possible to add bemotrizinol to the water phase.[6]
Bemotrizinol has strong synergistic effects on the SPF when formulated with bisoctrizole, ethylhexyl triazone or iscotrizinol.[7] It is the most effective UV absorber available measured by SPF, based on the maximum concentration permitted by European legislation.[8]
As of 2022,[9] bemotrizinol is not approved by the United States Food and Drug Administration for use in sunscreens, but has been approved in the European Union since 2000[10] and some other parts of the world, including Australia.[11][12]
Unlike some other organic sunscreen actives, it shows no estrogenic effects in vitro.[13]
References
- ↑ "Home". http://whqlibdoc.who.int/druginfo/18_4_2004_INN92.pdf.
- ↑ "Home". http://whqlibdoc.who.int/druginfo/INN_2005_list54.pdf.
- ↑ "Archived copy". http://www.ama-assn.org/ama1/pub/upload/mm/365/bemotrizinol.doc.
- ↑ "Sunscreens with an absorption maximum of > or =360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts". Photochem Photobiol Sci 5 (3): 275–82. March 2006. doi:10.1039/b516702g. PMID 16520862.
- ↑ "Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter". Photochem Photobiol 74 (3): 401–6. September 2001. doi:10.1562/0031-8655(2001)074<0401:POBMAA>2.0.CO;2. PMID 11594052.
- ↑ "basf-chemtrade.de". http://www.basf-chemtrade.de/images/stories/broschueren/PCI/100413_tinosorb_s_aqua.pdf.
- ↑ "BASF – Global Home". http://www.ciba.com/tinosorb-s_brochure.pdf. [|permanent dead link|dead link}}]
- ↑ "Study of the efficacy of 18 sun filters authorized in European Union tested in vitro". Pharmazie 62 (6): 449–52. June 2007. doi:10.1691/ph.2007.6.6247. PMID 17663193.
- ↑ Mull, Amanda (1 July 2022). "You're Not Allowed to Have the Best Sunscreens in the World" (in en). The Atlantic. https://www.theatlantic.com/technology/archive/2022/07/us-sunscreen-ingredients-outdated-technology-better-eu-asia/661433/.
- ↑ Osterwalder, U.; Luther, H.; Herzog, B. (2001). "Über den Lichtschutzfaktor hinaus - neue effiziente und photostabile UVA-Filter". Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 44 (5): 463–470. doi:10.1007/s001030170019.
- ↑ "NEW-WAVE SUNSCREENS: Active ingredient makers are frustrated by the long list of sunscreens and UV-A testing protocols that are still awaiting FDA decisions". Chemical & Engineering News 83 (15): 18–22. April 11, 2005. doi:10.1021/cen-v083n015.p018. http://pubs.acs.org/cen/coverstory/83/8315sunscreens.html.
- ↑ "Australian Regulatory Guidelines for OTC Medicines - Chapter 10". Archived from the original on August 31, 2007. https://web.archive.org/web/20070831075404/http://tga.gov.au/docs/pdf/argom_10.pdf.
- ↑ "Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S". Regul Toxicol Pharmacol 34 (3): 287–91. December 2001. doi:10.1006/rtph.2001.1511. PMID 11754532.
 |
