Chemistry:Avobenzone
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-(4-tert-Butylphenyl)-1-(4-methoxyphenyl)propane-1,3-dione | |
| Other names
butylmethoxydibenzoylmethane; 4-tert-butyl-4'-methoxydibenzoylmethane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
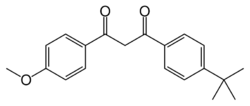
| C20H22O3 | |
| Molar mass | 310.39 g/mol |
| Appearance | colorless crystal |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Avobenzone (trade names Parsol 1789, Milestab 1789, Eusolex 9020, Escalol 517, Neo Heliopan 357 and others, INCI Butyl Methoxydibenzoylmethane) is an organic molecule and an oil-soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays.
History
Avobenzone was patented in 1973 and was approved in the EU in 1978. It was approved by the FDA in 1988. As of 2021, the FDA announced that they do not support avobenzone as being generally recognized as safe and effective (GRASE)[1] citing the need for additional safety data. Avobenzone was banned in 2020 by the Palau government citing toxicity concerns.[2]
Properties
Pure avobenzone is a whitish to yellowish crystalline powder with a weak odor,[3] dissolving in isopropanol, dimethyl sulfoxide, decyl oleate, capric acid/caprylic, triglycerides and other oils. It is not soluble in water.
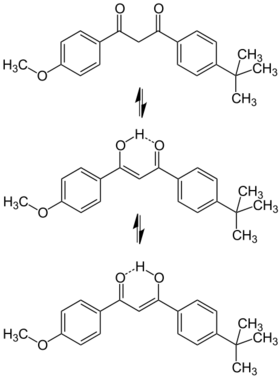
Avobenzone is a dibenzoylmethane derivative. Avobenzone exists in the ground state as a mixture of the enol and keto forms, favoring the chelated enol.[4] This enol form is stabilized by intramolecular hydrogen-bonding within the β-diketone.[5] Its ability to absorb ultraviolet light over a wider range of wavelengths than many other sunscreen agents has led to its use in many commercial preparations marketed as "broad spectrum" sunscreens. Avobenzone has an absorption maximum of 357 nm.[6]
Safety
Avobenzone, a petroleum-based sunscreen active ingredient,[7] is not generally recognised as safe and effective (GRASE) by the FDA for lack of sufficient data to support this claim.[1] However, it is still the only FDA approved UVA filter (up to 3% concentration).[8] Avobenzone is also approved in all other jurisdictions, such as EU (up to 5%), Australia , and Japan .
A 2017 study at Lomonosov Moscow State University found that chlorinated water and ultraviolet light can cause avobenzone to disintegrate into various other organic compounds, including; aromatic acids, aldehydes, phenols, and acetophenones which can cause adverse health effects.[9][10][11]
Stability
Avobenzone is sensitive to the properties of the solvent, being relatively stable in polar protic solvents and unstable in nonpolar environments. Also, when it is irradiated with UVA light, it generates a triplet excited state in the keto form which can either cause the avobenzone to degrade or it can transfer energy to biological targets and cause deleterious effects.[4]
Avobenzone has been shown to degrade significantly in light, resulting in less protection over time.[12][13][14] The UV-A light in a day of sunlight in a temperate climate is sufficient to break down most of the compound. Data presented to the Food and Drug Administration by the Cosmetic, Toiletry and Fragrance Association indicates a −36% change in avobenzone's UV absorbance following one hour of exposure to sunlight.[15] For this reason, in sunscreen products, avobenzone is always formulated together with a photostabilizer, such as octocrylene. Other photostabilizers include:
- 4-Methylbenzylidene camphor (USAN Enzacamene)
- Tinosorb S (USAN Bemotrizinol, INCI Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine)
- Tinosorb M (USAN Bisoctrizole, INCI Methylene Bis-Benzotriazolyl Tetramethylbutylphenol)
- Butyloctyl Salicylate (Tradename HallBrite BHB - [1])
- Hexadecyl Benzoate
- Butyloctyl Benzoate
- HallBrite PSF (INCI Undecylcrylene DimethiconeE)[16]
- Mexoryl SX (USAN Ecamsule, INCI Terephthalylidene Dicamphor Sulfonic Acid)
- Synoxyl HSS (INCI Trimethoxybenzylidene Pentanedione)[17]
- Corapan TQ (INCI Diethylhexyl 2,6-Naphthalate)[18]
- Parsol SLX (INCI Polysilicone-15)[19]
- Oxynex ST (INCI Diethylhexyl Syringylidene Malonate[20]
- Polycrylene (INCI Polyester-8)[21]
- SolaStay S1 (INCI Ethylhexyl Methoxycrylene)[22]
- Octyl Salicylate (INCI Ethylhexyl Salicylate)[23]
Complexing avobenzone with cyclodextrins may also increase its photostability.[24] Formulations of avobenzone with hydroxypropyl-beta-cyclodextrin have shown significant reduction in photo-induced degradation, as well as decreased transdermal penetration of the UV absorber when used in high concentrations.[25]
The photostability of avobenzone is further increased when sunscreens are formulated with antioxidant compounds. Mangiferin, glutathione, ubiquinone, vitamin C, vitamin E, beta-carotene and trans-resveratrol have all demonstrated some ability to protect avobenzone from photodegradation.[26][27][28][29] The stability and efficacy of avobenzone seems to continue to increase as a greater amount of antioxidants are added to the sunscreen.
According to some studies, "the most effective sunscreens contain avobenzone and titanium dioxide."[30][31] Avobenzone can degrade faster in light in combination with mineral UV absorbers like zinc oxide and titanium dioxide, though with the right coating of the mineral particles this reaction can be reduced.[32] A manganese doped titanium dioxide may be better than undoped titanium dioxide to improve avobenzone's stability.[33]
Various
As an enolate, avobenzone forms with heavy metal ions (such as Fe3+) colored complexes, and chelating agents can be added to suppress them. Stearates, aluminum, magnesium and zinc salts can lead to poorly soluble precipitates.[3] Manufacturers also recommend to avoid the inclusion of iron and ferric salts, heavy metals, formaldehyde donors and PABA and PABA esters.[citation needed]
Avobenzone in sunscreen may stain clothes yellow-orange and make them sticky if washed in iron-rich water, as it reacts with iron to produce rust. The damage can be undone with a rust remover or stain remover.[34][35] The staining properties of sunblock made with avobenzone are particularly noticeable on fiberglass boats with white gelcoat.[citation needed]
Avobenzone also reacts with boron trifluoride to form a stable crystalline complex that is highly fluorescent under UV irradiation. The emission color of the crystals depends on the molecular packing of the boron avobenzone complex. The photoluminescence may also be altered by mechanical force in the solid state, resulting in a phenomenon called "mechanochromic luminescence". The altered emission color recovers itself slowly at room temperature or more swiftly at higher temperatures.[36]
Absorbance spectrum
Avobenzone has a peak absorbance around 360 nm when dissolved. The peak may shift slightly depending on the solvent.

Preparation
The compound is prepared by reacting 4-tert-butylbenzoic methyl ester (from 4-tert-butylbenzoic acid by esterification with methanol) with 4-methoxyacetophenone in toluene in the presence of sodium amide via Claisen condensation.[37]
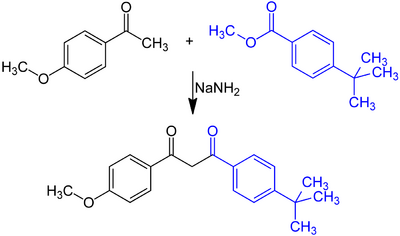
According to a recent patent application,[38] yields of up to 95% are obtained with the same starting materials in toluene in the presence of potassium methoxide.
It is subject to keto-enol tautomerism and exists predominantly enol when dissolved. Upon UV radiation, it may convert to keto form, while converting back to enol form after placing in dark.[39]
See also
- Sun protection factor
Notes
- ↑ 1.0 1.1 Research, Center for Drug Evaluation and (2021-11-16). "Questions and Answers: FDA posts deemed final order and proposed order for over-the-counter sunscreen" (in en). FDA. https://www.fda.gov/drugs/understanding-over-counter-medicines/questions-and-answers-fda-posts-deemed-final-order-and-proposed-order-over-counter-sunscreen.
- ↑ "REGULATIONS PROHIBITING REEF-TOXIC SUNSCREENS". The Palau Government. 2020. https://www.palaugov.pw/wp-content/uploads/2020/03/Sunscreen-Regulations-2020.pdf.
- ↑ 3.0 3.1 "Making Cosmetics®, Avobenzone". http://www.makingcosmetics.com/Avobenzone_p_258.html.
- ↑ 4.0 4.1 "A Blocked Diketo Form of Avobenzone: Photostability, Photosensitizing Properties and Triplet Quenching by a Triazine-derived UVB-filter". Photochemistry and Photobiology 85 (1): 178–184. January–February 2009. doi:10.1111/j.1751-1097.2008.00414.x. PMID 18673327.
- ↑ "Influence Of Substituent On UV Absorption And Keto–Enol Tautomerism Equilibrium Of Dibenzoylmethane Derivatives". Spectrochimica Acta Part A: Molecular Spectroscopy 96: 815–819. October 2012. doi:10.1016/j.saa.2012.07.109. PMID 22925908. Bibcode: 2012AcSpA..96..815Z.
- ↑ "Sunscreens with an absorption maximum of > or =360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts". Photochem Photobiol Sci 5 (3): 275–282. March 2006. doi:10.1039/b516702g. PMID 16520862.
- ↑ "Why Evaluate the Sunscreen Active Oxybenzone (Benzophenone-3) for Carcinogenicity and Reproductive Toxicology or Consider it Unsafe for Human Use?". https://www.scivisionpub.com/pdfs/why-evaluate-the-sunscreen-active-oxybenzone-benzophenone3-for-carcinogenicity-and-reproductive-toxicology-or-consider-it-unsafe-f-932.pdf.
- ↑ "After More Than A Decade, FDA Still Won't Allow New Sunscreens". https://cen.acs.org/articles/93/i20/Decade-FDA-Still-Wont-Allow.html.
- ↑ "Sunscreen creams break down into dangerous chemical compounds under the sunlight" (in en). https://www.eurekalert.org/pub_releases/2017-06/lmsu-scb062717.php.
- ↑ Wang, Cheng; Bavcon Kralj, Mojca; Košmrlj, Berta; Yao, Jun; Košenina, Suzana; Polyakova, Olga V.; Artaev, Viatcheslav B.; Lebedev, Albert T. et al. (September 2017). "Stability and removal of selected avobenzone's chlorination products". Chemosphere 182: 238–244. doi:10.1016/j.chemosphere.2017.04.125. PMID 28500968. Bibcode: 2017Chmsp.182..238W.
- ↑ Trebše, Polonca; Polyakova, Olga V.; Baranova, Maria; Kralj, Mojca Bavcon; Dolenc, Darko; Sarakha, Mohamed; Kutin, Alexander; Lebedev, Albert T. (2016-09-15). "Transformation of avobenzone in conditions of aquatic chlorination and UV-irradiation". Water Research 101: 95–102. doi:10.1016/j.watres.2016.05.067. PMID 27258620. Bibcode: 2016WatRe.101...95T.
- ↑ "Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter". Photochemistry and Photobiology 74 (3): 401–406. September 2001. doi:10.1562/0031-8655(2001)074<0401:POBMAA>2.0.CO;2. ISSN 0031-8655. PMID 11594052.
- ↑
 "Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation". Journal of Investigative Dermatology 113 (4): 547–553. October 1999. doi:10.1046/j.1523-1747.1999.00721.x. PMID 10504439.
"Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation". Journal of Investigative Dermatology 113 (4): 547–553. October 1999. doi:10.1046/j.1523-1747.1999.00721.x. PMID 10504439.
- ↑ "A new long-chain UV absorber derived from 4-tert-butyl-4'-methoxydibenzoylmethane: absorbance stability under solar irradiation". Journal of Cosmetic Science 56 (2): 135–148. Mar–Apr 2005. doi:10.1562/2004-03-09-ra-106. PMID 15870853.
- ↑ "CTFA letter re: Tentative Final Monograph for OTC Sunscreen". Food and Drug Administration. https://www.fda.gov/ohrms/dockets/dailys/00/Sep00/090600/c000573_10_Attachment_F.pdf.
- ↑ "Hallbrite® PSF". http://www.hallstar.com/pis.php?product=1A054.
- ↑ RK Chaudhuri, MA Ollengo, P Singh and BS Martincigh, 3-(3,4,5-Trimethoxybenzylidene)-2,4-pentanedione: Design of a Novel Photostabilizer with In-vivo SPF Boosting Properties and Its Use in Developing Broad-spectrum Sunscreen Formulations, International Journal of Cosmetic Science, 39(1):25-35, 2017; First published 29 June 2016 | doi: 10.1111/ics.12344
- ↑ Bonda C.; Steinberg D. C. (2000). "A new photostabilizer for full spectrum sunscreens". Cosmetics & Toiletries 115 (6): 37–45.
- ↑ "Archived copy". http://www.dsm.com/en_US/downloads/dnp/Parsol_SLX_Skin.pdf.
- ↑ "Design of a photostabilizer having built-in antioxidant functionality and its utility in obtaining broad-spectrum sunscreen formulations". Photochemistry and Photobiology 82 (3): 823–828. May–Jun 2006. doi:10.1562/2005-07-15-RA-612. PMID 16492073.
- ↑ http://www.hallstar.com/techdocs/Polycrylene&CorapanTQAvobenzoneStabilization.pdf [bare URL PDF]
- ↑ "Product Information Sheet: SolaStay S1". The HallStar Company. http://www.hallstar.com/pis.php?product=1H044.
- ↑ Ma, Haohua; Wang, Jianqiang; Zhang, Wenpei; Guo, Cheng (2021). "Synthesis of phenylalanine and leucine dipeptide functionalized silica-based nanoporous material as a safe UV filter for sunscreen". Journal of Sol-Gel Science and Technology 97 (2): 466–478. doi:10.1007/s10971-020-05417-6. https://link.springer.com/article/10.1007/s10971-020-05417-6.
- ↑ "Influence of hydroxypropyl-beta-cyclodextrin on photo-induced free radical production by the sunscreen agent, butyl-methoxydibenzoylmethane". Journal of Pharmacy and Pharmacology 54 (11): 1553–1558. November 2002. doi:10.1211/002235702207. PMID 12495559.
- ↑ "Influence of hydroxypropyl-β-cyclodextrin on transdermal penetration and photostability of avobenzone". European Journal of Pharmaceutics and Biopharmaceutics 69 (2): 605–612. June 2008. doi:10.1016/j.ejpb.2007.12.015. PMID 18226883.
- ↑ Kawakami, Camila Martins; Gaspar, Lorena Rigo (October 2015). "Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone". Journal of Photochemistry and Photobiology B: Biology 151: 239–247. doi:10.1016/j.jphotobiol.2015.08.014. ISSN 1873-2682. PMID 26318281. https://pubmed.ncbi.nlm.nih.gov/26318281/.
- ↑ Govindu, Panchada Ch V.; Hosamani, Basavaprabhu; Moi, Smriti; Venkatachalam, Dhananjeyan; Asha, Sabreddy; John, Varun N.; Sandeep, V.; Gowd, Konkallu Hanumae (2019-01-01). "Glutathione as a photo-stabilizer of avobenzone: an evaluation under glass-filtered sunlight using UV-spectroscopy". Photochemical & Photobiological Sciences 18 (1): 198–207. doi:10.1039/c8pp00343b. ISSN 1474-9092. PMID 30421772.
- ↑ Afonso, S.; Horita, K.; Sousa e Silva, J. P.; Almeida, I. F.; Amaral, M. H.; Lobão, P. A.; Costa, P. C.; Miranda, Margarida S. et al. (November 2014). "Photodegradation of avobenzone: stabilization effect of antioxidants". Journal of Photochemistry and Photobiology B: Biology 140: 36–40. doi:10.1016/j.jphotobiol.2014.07.004. ISSN 1873-2682. PMID 25086322. https://pubmed.ncbi.nlm.nih.gov/25086322/.
- ↑ Freitas, Juliana Vescovi; Lopes, Norberto Peporine; Gaspar, Lorena Rigo (2015-10-12). "Photostability evaluation of five UV-filters, trans-resveratrol and beta-carotene in sunscreens". European Journal of Pharmaceutical Sciences 78: 79–89. doi:10.1016/j.ejps.2015.07.004. ISSN 1879-0720. PMID 26159738. https://pubmed.ncbi.nlm.nih.gov/26159738/.
- ↑ Warwick L. Morison, M.D. (March 11, 2004). "Photosensitivity". The New England Journal of Medicine 350 (11): 1111–1117. doi:10.1056/NEJMcp022558. PMID 15014184.
- ↑ "Sunscreen Drug Products for Over-the-Counter use; Marketing Status of Products Containing Avobenzone; Enforcement Policy". US Food and Drug Administration. 1997-04-30. p. 23354. https://www.fda.gov/cder/otcmonographs/Sunscreen/sunscreen_avobenzone_enforc_policy_19970420.pdf.
- ↑ Stability Study of Avobenzone with Inorganic Sunscreens, Kobo Products Poster, 2001, Online version
- ↑ "The effects of manganese doping on UVA absorption and free radical generation of micronised titanium dioxide and its consequences for the photostability of UVA absorbing organic sunscreen components". Photochem Photobiol Sci 3 (7): 648–652. July 2004. doi:10.1039/b403697b. PMID 15238999.
- ↑ "How to Remove Summer Stains From Your Clothes". Consumer Reports. 17 August 2022. https://www.consumerreports.org/appliances/laundry/how-to-remove-summer-stains-from-your-clothes-a3477204035/.
- ↑ "The secret to removing sunscreen stains is this unexpected cleaning product". CNN. 18 July 2022. https://edition.cnn.com/cnn-underscored/home/how-to-remove-sunscreen-stains.
- ↑ Zhang G; Lu J; Sabat M; Fraser, CL (February 2010). "Polymorphism and Reversible Mechanochromic Luminescence for Solid-State Difluoroboron Avobenzone". Journal of the American Chemical Society 132 (7): 2160–2162. doi:10.1021/ja9097719. PMID 20108897.
- ↑ US patent 0
- ↑ US patent 0
- ↑ G. J. Mturi, B. S. Martincigh (2008), "Photostability of the sunscreening agent 4-tert-butyl-4-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity", J. Photochem. Photobiol.: Chemistry 200 (2–3): 410–420, doi:10.1016/j.jphotochem.2008.09.007
 |