Chemistry:Octocrylene
| |
| |
| Names | |
|---|---|
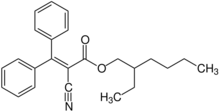
| Preferred IUPAC name
2-Ethylhexyl 2-cyano-3,3-diphenylprop-2-enoate | |
| Other names
Octocrylene
Octocrilene Uvinul N-539 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C24H27NO2 | |
| Molar mass | 361.48 g/mol |
| Density | 1.05 g/cm3 |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 218 °C (424 °F; 491 K) at 1.5 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octocrylene is an organic compound used as an ingredient in sunscreens and cosmetics. It is an ester formed by the condensation of 2-ethylhexyl cyanoacetate with benzophenone. It is a viscous, oily liquid that is clear and colorless.
The extended conjugation of the acrylate portion of the molecule absorbs UVB and short-wave UVA (ultraviolet) rays with wavelengths from 280 to 320 nm,[1] protecting the skin from direct DNA damage. The ethylhexanol portion is a fatty alcohol, adding emollient and oil-like (water resistant) properties.
Safety
Octocrylene can penetrate into the skin where it acts as a photosensitizer, resulting in an increased production of free radicals under illumination.[2] It may also pass through the skin, into the blood stream, eventually being metabolized and excreted in urine in form of its metabolites.[3][4] Octocrylene can convert to benzophenone through a retro-aldol condensation.[5] The reaction occurs slowly over time, yielding significant concentration of benzophenone in all commercial cosmetics tested formulated with octocrylene.
In coral, octocrylene has been shown to accumulate in the form of fatty acid conjugates and trigger mitochondrial dysfunction.[6]
Regulation
Palau has banned the sale and use of three reef-toxic UV filters including octocrylene in its Responsible Tourism Education Act of 2018.[7]
See also
- Sunscreen controversy
References
- ↑ Smart Skin Care: Octocrylene
- ↑ Hanson Kerry M.; Gratton Enrico; Bardeen Christopher J. (2006). "Sunscreen enhancement of UV-induced reactive oxygen species in the skin". Free Radical Biology and Medicine 41 (8): 1205–1212. doi:10.1016/j.freeradbiomed.2006.06.011. PMID 17015167. http://www.escholarship.org/uc/item/9f14s2dd.
- ↑ Bury, Daniel; Belov, Vladimir N.; Qi, Yulin; Hayen, Heiko; Volmer, Dietrich A.; Brüning, Thomas; Koch, Holger M. (2018). "Determination of urinary metabolites of the emerging UV filter octocrylene by online-SPE-LC-MS/MS". Analytical Chemistry 90 (1): 944–951. doi:10.1021/acs.analchem.7b03996. PMID 29188988.
- ↑ Bury, Daniel; Modick-Biermann, Hendrik; Leibold, Edgar; Brüning, Thomas; Koch, Holger M. (2019). "Urinary metabolites of the UV filter octocrylene in humans as biomarkers of exposure". Archives of Toxicology 93 (5): 1227–1238. doi:10.1007/s00204-019-02408-7. PMID 30739143.
- ↑ Downs, C. A.; DiNardo, Joseph C.; Stien, Didier; Rodrigues, Alice M. S.; Lebaron, Philippe (7 March 2021). "Benzophenone Accumulates over Time from the Degradation of Octocrylene in Commercial Sunscreen Products". Chemical Research in Toxicology 34 (4): 1046–1054. doi:10.1021/acs.chemrestox.0c00461. PMID 33682414.
- ↑ Stien, Didier; Clergeaud, Fanny; Rodrigues, Alice M.S.; Lebaron, Karine; Pillot, Rémi; Romans, Pascal; Fagervold, Sonja; Lebaron, Philippe (2019). "Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction". Analytical Chemistry 91 (1): 990–995. doi:10.1021/acs.analchem.8b04187. PMID 30516955. https://hal.sorbonne-universite.fr/hal-01973836/file/Manuscript%20File.pdf.
- ↑ Republic of Palau. "RPPL No. 10-30: The Responsible Tourism Education Act of 2018 – PalauGov.pw". https://www.palaugov.pw/documents/rppl-no-10-30-the-responsible-tourism-education-act-of-2018/.
 |
