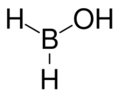
Chemistry:Borinic acid
| |
| Names | |
|---|---|
| IUPAC name
Borinic acid
| |
| Systematic IUPAC name
Boranol | |
| Other names
Boranol, Hydroxyborane, Dihydridohydroxidoboron
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 141192 | |
PubChem CID
|
|
| |
| |
| Properties | |
| BH3O | |
| Molar mass | 29.83 g·mol−1 |
| reaction in water | |
| Structure | |
| distorted trigonal bipyramid | |
| Related compounds | |
Related compounds
|
Boronic acid Boric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Borinic acid, also known as boronous acid, is an oxyacid of boron with formula H2BOH. Borinate is the associated anion of borinic acid with formula H2BO−; however, being a Lewis acid, the form in basic solution is H2B(OH)−2.
Borinic acid can be formed as the first step in the hydrolysis of diborane:[1]
- BH3 + H2O → H2BOH + H2
Borinic acid itself is unstable and only lasts for a few seconds during the hydrolysis reaction. However, by using microwave spectroscopy various properties can be determined. The B-O distance is 1.352 Å, O-H distance 0.96 Å, B-H length is probably 1.2 Å. The angle between bonds at the oxygen atom BOH = 112° and the angles at boron are cis-HBO 121°, and trans-HBO = 117°. The dipole moment is 1.506 Debye.[2]
Borinic acid can form esters such as methoxyborane. This too is unstable only lasting about ten seconds. It can be formed by heating diborane and methanol gas together.[3]
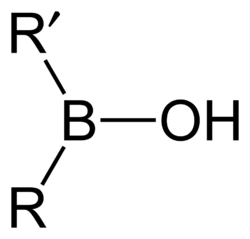
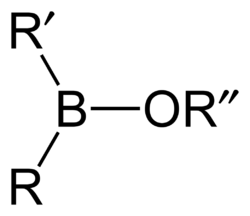
By substituting organic components instead of hydrogen, more generic borinic acids (containing RR'BOH) or borinic esters (RR'BOR") can be formed. Esters will tend to be stable in acidic conditions, but in alkaline conditions the boron atom can gain a negative charge and attach two hydroxyl groups, or two ester bonds. RR'B−(OH)2 or RR'B−(OR")2. The anionic borinate ion can very easily form esters with diols such as ethylene glycol or sugars.[citation needed]
Naming
The IUPAC name borinic acid is a unique name for the acid.[4] The anhydrides are named diboroxanes, H2BOBH2, as the base compound and H being able to be substituted, e.g. tetraethyldiboroxane, as the anhydride for diethylborinic acid. Organic naming standard in the Bluebook allows skeletal replacement naming where the name is shorter, 3-borapentan-3-ol versus diethyl borinic acid. The grouping -BH-O-BH2 is called diboroxanyl. Substituting sulfur for oxygen gives borinothioic acid (H2BSH). (Dimethylboranyl)oxy is used for the group (CH3)2B-O− and methyl(hydroxy)boranyl for the grouping CH3B(OH)-.
Formation
There are several ways to produce substituted borinic acids.[5]
Firstly borinic acids can be made from oxidizing trialkyl borane starting materials [R3B] with exposure to moist air, or treatment with iodine, which makes a dialkyliodoborane [R2BI]. Hydrolysis then results in the boronic acid (R2BOH).[5] Trialkylborates [(RO)3B] or trialkoxyboroxine [(ROBO)3] can be reduced to borinic acid by us of a Grignard reagent. Grignard reagents can also reduce a boronic ester [RB(OR')2] to a borinic ester.[5]
- Bu3B + N2CHCOR → BuCH=C(R)OBBu2
- Bu3B + CH2=CHCOCH3 → BuCH2CH=C(CH3)OBBu2
- RCOC2H5 + R2BOTf → RC(OBR2)=CHCH3
- (Tf = Trifluoromethanesulfonate)
(Z)-Enolates give syn aldol product when reacted with an aldehyde, whereas (E)-enolates give and anti aldol products.
Dialkyl boron chloride (R2BCl) with tertiary amine react with ketones to form an enol borinate.[6]
A trialkoxyborane can react with lithium containing organic molecules to eliminate lithium and one or two alkoxy groups to make boronic and borinic esters.[7]
Purification of the mixtures that result from the reactions is required, as often boronic esters will also be produced and mixed in with the borinic esters.[5] The method of Letsinger is to dissolve the mixture in ether and precipitate the borinic ester by forming a complex with ammonia. Treatment with ethanolamine ends up making an aminoetylborinate.[5]
Compounds
| R2BOR' | borinic acid R'=H | anhydride | esters R' | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | hydrogen | -O- | aminoethyl | ethyl | n-propyl | n-butyl | 3-methylpropyl | 1-methylpropyl | phenyl | ethylene glycol | methyl | 8-quinolinyl |
| phenyl | [8] | SID 535455 | ||||||||||
| o-tolyl | ||||||||||||
| m-tolyl | ||||||||||||
| p-tolyl | ||||||||||||
| p-anisyl | ||||||||||||
| p-biphenyl | ||||||||||||
| p-chlorophenyl | ||||||||||||
| 3-chlorophenyl | ||||||||||||
| α-naphthyl | ||||||||||||
| β-naphthyl | ||||||||||||
| p-bromophenyl | ||||||||||||
| 2-methyl-5-chlorophenyl | ||||||||||||
| 2-thienyl | [16] SID 3881207 | SID 8142470 | ||||||||||
| mesityl | sid 4278417 CAS 20631-84-9[17][18][19] | |||||||||||
| methyl | ||||||||||||
| ethyl | ||||||||||||
| allyl | ||||||||||||
| n-butyl | cas 19324-14-2 | |||||||||||
| 4-methylbutyl | ||||||||||||
| 2-chlorovinyl | ||||||||||||
| 3,5-dimethylphenyl | ||||||||||||
| propyl | ||||||||||||
| 1-methylpropyl | cas 4026-69-1 | |||||||||||
| 2-methylpropyl | cas 4026-82-8 | |||||||||||
2-APB
2-Aminoethoxydiphenyl borate (2-APB) inhibits transient receptor potential channels.[24] This kind of inhibition, particularly inhibition of TRPM7, is being studied to find treatments for prostate cancer. 2-APB can work as a catalyst to add an alkyl group from an alkyl halide to a polyol or carbohydrate that contains a cis-vicinal diol to a precise position. It does this by first combining with the two hydroxy groups to make a ring containing OCCOB−.[25] It can also calatlyse acid chloride or chloroformate reaction a specific region of the diol.[26]
Diphenylborinic acid
Diphenylborinic acid was discovered in 1894 by Michaelis who produced it by hydrolyzing the chloride. Letsinger determined its properties in 1955.[5]
Diphenylborinic acid has an extra high affinity for catechols compared with carbohydrates.[27]
Diphenylborinic acid can catalyse the condensation of pyruvic acids with aldehydes to yield substituted isotetronic acid.[28]
Diphenylborinic acid is an inhibitor of several enzymes such as α-chymotrypsin, subtilisin BPN', and trypsin.[29]
Borinate radical
Borinate radicals (RR'BO·) can be formed from peroxyborinate decomposition.[30]
Other compounds
Other compounds include methoxy(dimethyl)borane, methoxy(methyl)boron, methoxy(methylidene)borane (with a C=B double bond).[31]
[C5H5BR]− uses a B− to be equivalent to carbon in an aromatic benzene like ring. This too is called borinate. The 1-methyl and 1-phenyl borinates can form some of the few organo-thallium(I) compounds.[32]
HB(C6F5)2 + phosphino alcohol → tBu2P+HCH2C(CH3)2OB−H(C6F5)2 → H2 + tBu2PCH2C(CH3)2OB(C6F5)2 and same for tBu2PCH2C(CF3)2OB(C6F5)2[33]
di-Tris(tert-butoxy)siloxy borinic acid HOB[OSi(O(t)Bu)3]2 can be made from tributoxyborate and tributoxysiloxane. It can form a very complex crystal with Cp2Zr(Me)[OB[OSi(O(t)Bu)3]2]2.[34]
Diborinic acids have two RBOH groups linked together by an organic connection such as diphenyl or phenyl.[35]
Applications
1,1,1,3,3,3-Hexafluoroisopropylbis(pentafluorophenyl)borinate can greatly increase solubility of LiF by complexing the F− anion.[36] This has potential to improve lithium batteries.
Borinic esters are being researched as bacterial growth inhibitors[37] due to their ability to disable some bacterial enzymes such as menaquinone methyltransferase and CcrM.[38] This may result in development of treatments for topical application on skin.[39]
References
- ↑ Weiss, H. G.; Shapiro, I. (5 March 1953). "Mechanism of the Hydrolysis of Diborane in the Vapor Phase1". Journal of the American Chemical Society 75 (5): 1221–1224. doi:10.1021/ja01101a061.
- ↑ Kawashima, Yoshiyuki; Takeo, Harutoshi; Matsumura, Chi (1 January 1981). "Microwave spectrum of borinic acid BH2OH". The Journal of Chemical Physics 74 (10): 5430. doi:10.1063/1.440947. Bibcode: 1981JChPh..74.5430K.
- ↑ Kawashima, Y.; Takeo, H.; Matsumura, C. (1984). Microwave Spectrum of Methoxyborane, CH3OBH2. https://hdl.handle.net/1811/16666.
- ↑ Connelly, Neil G.; Damhus, Ture; Hartshorn, Richard M.; Alan T. Hutton (2005). "Table IR-8.1 Acceptable common names". Nomenclature of Inorganic Chemistry. International Union of Pure and Applied Chemistry. p. 127. ISBN 0-85404-438-8.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf.
- ↑ Brown, Herbert C; Rhaj K. Dhar (March 16, 1989). "Major Effect of the Leaving Group in Dialkyl Boron Chlorides and Triflates in COntrolling the Stereoscopic COnversion of Ketones into Either [E or [Z]- Enol Borinates"]. http://www.dtic.mil/dtic/Ftr/fulltext/u2/a209988.pdf.
- ↑ Delbrayelle, Dominique (15 June 2010). "Boronic Acids Manufacture at Industrial Scale". http://www.chemspecevents.com/europe/images/uploads/chemsource/10-5_Delbrayelle.pdf.
- ↑ Lange, Thomas; Böhme, Uwe; Roewer, Gerhard (2002). "Crystal structure of tetraphenyldiboroxane a monomer diboroxane". Inorganic Chemistry Communications 5 (6): 377–379. doi:10.1016/S1387-7003(02)00402-1.
- ↑ Erbes, Michael; Klaus Forstinger (31 Oct 2002). "Process for preparing boronic and borinic acids". US Patent 20020161230. http://www.google.com.au/patents/US20020161230.
- ↑ 10.0 10.1 10.2 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 24
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 25
- ↑ 12.0 12.1 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 26
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 5
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 9
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 2
- ↑ Low, John Nicolson; Musgrave, Oliver; Wardell, James (15 February 2000). "(2-Aminoethoxy)bis(2-thienyl)boron". Acta Crystallographica Section C 56 (2): e63. doi:10.1107/S0108270100000482. Bibcode: 2000AcCrC..56E..63L.
- ↑ Birch, Samuel J.; Boss, Sally R.; Cole, Sarah C.; Coles, Martyn P.; Haigh, Robert; Hitchcock, Peter B.; Wheatley, Andrew E. H. (1 January 2004). "The structural characteristics of organozinc complexes incorporating N,N?-bidentate ligands". Dalton Transactions (21): 3568–3574. doi:10.1039/B410945G. PMID 15510278.
- ↑ Kuhlmann, Matthias; Baumgartner, Thomas; Parvez, Masood (7 June 2008). "A new polymorph of dimesitylborinic acid". Acta Crystallographica Section E 64 (7): o1185. doi:10.1107/S1600536808015638. PMID 21202827. Bibcode: 2008AcCrE..64O1185K.
- ↑ Weese, Kenneth J.; Bartlett, Ruth A.; Murray, Brendan D.; Olmstead, Marilyn M.; Power, Philip R. (1 July 1987). "Synthesis and spectroscopic and structural characterization of derivatives of the quasi-alkoxide ligand [OBMes2]- (Mes = 2,4,6-Me3C6H2)". Inorganic Chemistry 26 (15): 2409–2413. doi:10.1021/ic00262a015.
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 28
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 17
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability". http://ufdcimages.uflib.ufl.edu/UF/00/09/15/96/00001/Binder1.pdf. ref 29
- ↑ Winkle, D. (2001). "boronic acids can be used as viable alternatives to borinic acids in suzuki coupling reactions". Organic Process Research & Development 5 (4): 450–451. doi:10.1021/op010207s. https://www.scientificupdate.co.uk/process-chemistry-information-sp-1242612461/item/boronic-acids-can-be-used-as-viable-alternatives-to-borinic-acids-in-suzuki-coupling-reactions.html.
- ↑ Carboxylic Acids—Advances in Research and Application. ScholarlyEditions. 2012. p. 93. ISBN 978-1-4649-9392-3. https://books.google.com/books?id=dpCfvweP9uUC&pg=PA93.
- ↑ Chan, Lina; Taylor, Mark S. (17 June 2011). "Regioselective Alkylation of Carbohydrate Derivatives Catalyzed by a Diarylborinic Acid Derivative". Organic Letters 13 (12): 3090–3093. doi:10.1021/ol200990e. PMID 21591630.
- ↑ Lee, Doris; Taylor, Mark S. (23 March 2011). "Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives". Journal of the American Chemical Society 133 (11): 3724–3727. doi:10.1021/ja110332r. PMID 21355584.
- ↑ Chudzinski, Michael G.; Chi, Yuechuan; Taylor, Mark S. (1 January 2011). "Borinic Acids: A Neglected Class of Organoboron Compounds for Recognition of Diols in Aqueous Solution". Australian Journal of Chemistry 64 (11): 1466. doi:10.1071/CH11294.
- ↑ Lee, Doris; Newman, Stephen G.; Taylor, Mark S. (3 December 2009). "Boron-Catalyzed Direct Aldol Reactions of Pyruvic Acids". Organic Letters 11 (23): 5486–5489. doi:10.1021/ol902322r. PMID 19904926.
- ↑ Steiner, Steven J.; Bien, Jeffrey T.; Smith, Bradley D. (1 October 1994). "Diphenylborinic acid is a strong inhibitor of serine proteases". Bioorganic & Medicinal Chemistry Letters 4 (20): 2417–2420. doi:10.1016/S0960-894X(01)80401-7.
- ↑ Robin Hicks (2 August 2011). Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. John Wiley and Sons. p. 518. ISBN 978-1-119-95696-9. https://books.google.com/books?id=WuwW8QDuCKIC&pg=PT518.
- ↑ "Similar compounds". https://www.ncbi.nlm.nih.gov/pccompound?LinkName=pccompound_pccompound&cmd=Link&from_uid=138247.
- ↑ Janiac (1997). "(Organo) thallium (I) and (II) chemistry: syntheses, structures, properties and applications of subvalent thallium complexes with alkyl, cyclopentadienyl, arene or hydrotris (pyrazolyl) borate ligands". Coordination Chemistry Reviews 163: 124. doi:10.1016/s0010-8545(97)00011-8. http://portal.uni-freiburg.de/janiak/veroffentlichungen/pdf/59.pdf/at_download/file.
- ↑ Chapman, Andy M.; Haddow, Mairi F.; Orton, Jonathan P. H.; Wass, Duncan F. (1 January 2010). "Facile dihydrogen release from phosphino-borinate ester Lewis pairs". Dalton Transactions 39 (27): 6184–6. doi:10.1039/c0dt00513d. PMID 20544115.
- ↑ Fujdala, KL; Oliver, A. G.; Hollander, F. J.; Tilley, T. D. (Feb 24, 2003). "Tris(tert-butoxy)siloxy derivatives of boron, including the boronous acid HOB[OSi(O(t)Bu)(3)](2) and the metal (siloxy)boryloxide complex Cp(2)Zr(Me)OB[OSi(O(t)Bu)(3)](2): a remarkable crystal structure with 18 independent molecules in its asymmetric unit". Inorganic Chemistry 42 (4): 1140–1150. doi:10.1021/ic0205482. PMID 12588150.
- ↑ Suzuki, Akinobu Z.; Shoichiro Ozaki, Jun-Ichi Goto Katsuhiko Mikishiba (15 Feb 2010). "Synthesis of bisboron Compounds and their Strong Inhibitory Activity on Store-operated Calcium Entry". Bioorganic & Medicinal Chemistry Letters 20 (4): 1395–1398. doi:10.1016/j.bmcl.2009.12.108. PMID 20097561.
- ↑ McBreen, J.; H. S. Lee (2003). "Borinates: A New Family of Anion Complexing Agents". The Electrochemical Society, Inc.. http://www.electrochem.org/dl/ma/204/Fpdfs/0298.pdf.
- ↑ Bailey, P. J.; G Cousins; G A Snow; A J White (April 1980). "Boron-containing antibacterial agents: effects on growth and morphology of bacteria under various culture conditions". Antimicrobial Agents and Chemotherapy (American Society for Microbiology) 17 (4): 549–553. doi:10.1128/aac.17.4.549. PMID 6994634.
- ↑ "Identification of Borinic Esters as Inhibitors of Bacterial Cell Growth and Bacterial Methyltransferases, CcrM and MenH". Journal of Medicinal Chemistry 48 (23): 7468–7476. 1 November 2005. doi:10.1021/jm050676a. PMID 16279806.
- ↑ "Identification of a novel boron-containing antibacterial agent (AN0128) with anti-inflammatory activity, for the potential treatment of cutaneous diseases". Bioorg. Med. Chem. Lett. 16 (23): 5963–7. 2006. doi:10.1016/j.bmcl.2006.08.130. PMID 16997550.
Extra reading
- Dimitrijević, Elena; Taylor, Mark S. (3 May 2013). "Organoboron Acids and Their Derivatives as Catalysts for Organic Synthesis". ACS Catalysis 3 (5): 945–962. doi:10.1021/cs4000848. Review of use borinic acids as a catalyst
- Roth, HJ; Miller, B (September 1964). "Synthesis of Phenyl- and Thienyl-Substituted Boronic and Borinic Acids". Archiv der Pharmazie 297 (9): 513–523. doi:10.1002/ardp.19642970902. PMID 14341914.
 |