Chemistry:Butyraldehyde

| |
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Butanal | |
| Other names
Butyraldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1129 |
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.107 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Pungent, aldehyde odor |
| Density | 0.8016 g/mL |
| Melting point | −96.86 °C (−142.35 °F; 176.29 K) |
| Boiling point | 74.8 °C (166.6 °F; 347.9 K) |
| Critical point (T, P) | 537 K (264 °C), 4.32 MPa (42.6 atm) |
| 7.6 g/100 mL (20 °C) | |
| Solubility | Miscible with organic solvents |
| log P | 0.88 |
| −46.08·10−6 cm3/mol | |
Refractive index (nD)
|
1.3766 |
| Viscosity | 0.45 cP (20 °C) |
| 2.72 D | |
| Thermochemistry[2] | |
Heat capacity (C)
|
163.7 J·mol−1·K−1 (liquid) 103.4 J·mol−1·K−1 (gas) |
Std molar
entropy (S |
246.6 J·mol−1·K−1 (liquid) 343.7 J·mol−1·K−1 (gas) |
Std enthalpy of
formation (ΔfH⦵298) |
−239.2 kJ·mol−1 (liquid) −204.8 kJ·mol−1 (gas) |
Std enthalpy of
combustion (ΔcH⦵298) |
2470.34 kJ·mol−1 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| GHS pictograms |   [3] [3]
|
| GHS Signal word | Danger |
| H225, H319[3] | |
| P280, P304+340, P302+352, P210, P305+351+338[3] | |
| NFPA 704 (fire diamond) | |
| Flash point | −7 °C (19 °F; 266 K) |
| 230 °C (446 °F; 503 K) | |
| Explosive limits | 1.9–12.5% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2490 mg/kg (rat, oral) |
| Related compounds | |
Related aldehyde
|
Propionaldehyde Pentanal |
Related compounds
|
Butan-1-ol Butyric acid, isobutyraldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
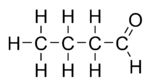
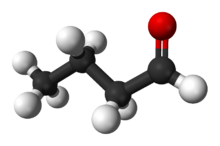
Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colorless flammable liquid with an unpleasant smell. It is miscible with most organic solvents.
Production
Butyraldehyde is produced almost exclusively by the hydroformylation of propylene:
- CH3CH=CH2 + H2 + CO → CH3CH2CH2CHO
Traditionally, hydroformylation was catalyzed by cobalt carbonyl and later rhodium complexes of triphenylphosphine. The dominant technology involves the use of rhodium catalysts derived from the water-soluble ligand tppts. An aqueous solution of the rhodium catalyst converts the propylene to the aldehyde, which forms a lighter immiscible phase. About 6 billion kilograms are produced annually by hydroformylation. Butyraldehyde can be produced by the catalytic dehydrogenation of n-butanol. At one time, it was produced industrially by the catalytic hydrogenation of crotonaldehyde, which is derived from acetaldehyde.[4]
Reactions
Butyraldehyde undergoes reactions typical of alkyl aldehydes, and these define many of the uses of this compound. Important reactions include hydrogenation to the alcohol, oxidation to the acid, and base-catalyzed condensation.
Uses
Aldol condensation in the presence of a base forms 2-ethyl-2-hexenal, which is then hydrogenated to form 2-ethylhexanol, a precursor to the plasticizer bis(2-ethylhexyl) phthalate.[4]
Butyraldehyde is a precursor in the two-step synthesis of trimethylolpropane, which is used for the production of alkyd resins.[5]

References
- ↑ Merck Index, 11th Edition, 1591.
- ↑ CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data.. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942. https://www.worldcat.org/oclc/930681942.
- ↑ 3.0 3.1 3.2 Record of Butyraldehyde in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 13 March 2020.
- ↑ 4.0 4.1 Raff, Donald K. (2013). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_447.pub2.
- ↑ Werle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2.
External links
 |

