Chemistry:Isobutyraldehyde
| |
| |
| Names | |
|---|---|
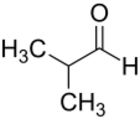
| Preferred IUPAC name
2-Methylpropanal | |
| Other names
2-Methylpropionaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605330 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 1658 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2045 |
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.11 g/mol |
| Appearance | colourless liquid |
| Odor | Pungent; straw-like |
| Density | 0.79 g/cm3 |
| Melting point | −65 °C (−85 °F; 208 K) |
| Boiling point | 63 °C (145 °F; 336 K) |
| moderate | |
| Solubility in other solvents | miscible in organic solvents |
| -46.38·10−6 cm3/mol | |
Refractive index (nD)
|
1.374 |
| Hazards | |
| Main hazards | flammable |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H225, H319 | |
| P210, P233, P240, P241, P242, P243, P264, P280, P303+361+353, P305+351+338, P337+313, P370+378, P403+235, P501 | |
| Flash point | −19 °C; −2 °F; 254 K |
| Related compounds | |
Related alkyl aldehydes
|
Lilial |
Related compounds
|
Butyraldehyde Propionaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isobutyraldehyde is the chemical compound with the formula (CH3)2CHCHO. It is an aldehyde, isomeric with n-butyraldehyde (butanal).[1] Isobutyraldehyde is made, often as a side-product, by the hydroformylation of propene. Its odour is described as that of wet cereal or straw. It undergoes the Cannizaro reaction even though it has alpha hydrogen atom. It is a colorless volatile liquid.
Synthesis
Isobutyraldehyde is produced industrially by the hydroformylation of propene. Several million tons are produced annually.[2]
Biological routes
In the context of butanol fuel, isobutyraldehyde is of interest as a precursor to isobutanol. E. coli as well as several other organisms has been genetically modified to produce isobutanol. α-Ketoisovalerate, derived from oxidative deamination of valine, is prone to decarboxylation to give isobutyraldehyde, which is susceptible to reduction to the alcohol:[3]
- (CH3)2CHC(O)CO2H → (CH3)2CHCHO + CO2
- (CH3)2CHCHO + NADH + H+ → (CH3)2CHCH2OH + NAD+
Other routes
It can also be produced using engineered bacteria.[4]
Strong mineral acids catalyse the rearrangement of methallyl alcohol to isobutyraldehyde.
Reactions
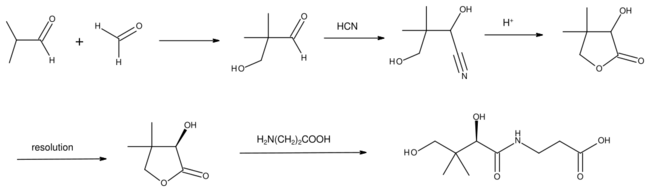
Hydrogenation of the aldehyde gives isobutanol. Oxidation gives methacrolein or methacrylic acid. Condensation with formaldehyde gives hydroxypivaldehyde.[2] The latter is a precursor to vitamin B5.[5]
References
- ↑ Isobutyraldehyde is a retained trivial name under the IUPAC rules.Panico, R.; Powell, W. H.; Richer, J. C., eds (1993). "Recommendation R-9.1". A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. ISBN 0-632-03488-2. https://www.acdlabs.com/iupac/nomenclature/.
- ↑ 2.0 2.1 Boy Cornils, Richard W. Fischer, Christian Kohlpaintner "Butanals" in Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_447
- ↑ Atsumi, Shota; Hanai, Taizo; Liao, James C. (January 2008). "Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels". Nature 451 (7174): 86–89. doi:10.1038/nature06450. PMID 18172501. Bibcode: 2008Natur.451...86A.
- ↑ Atsumi, Shota; Wendy Higashide; James C. Liao (November 2009). "Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde". Nature Biotechnology 27 (12): 1177–1180. doi:10.1038/nbt.1586. PMID 19915552.
- ↑ Eggersdorfer, Manfred; Laudert, Dietmar; Létinois, Ulla; McClymont, Tom; Medlock, Jonathan; Netscher, Thomas; Bonrath, Werner (2012). "One Hundred Years of Vitamins-A Success Story of the Natural Sciences". Angewandte Chemie International Edition 51 (52): 12975. doi:10.1002/anie.201205886. PMID 23208776.
 |