Chemistry:Cerium(III) selenate
From HandWiki
File:3.svg 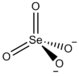
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Ce2(SeO4)3 | |
| Molar mass | 709.121 |
| Density | 4.456g/cm3 (octahydrate)[1] |
| 39.5g(0 °C) 2.51(100 °C) | |
| Related compounds | |
Other anions
|
cerium(III) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cerium(III) selenate is an inorganic compound with the chemical formula Ce
2(SeO
4)
3. It can be obtained by reacting selenic acid and cerium(III) carbonate, and the solvent is evaporated to precipitate crystals.[2] The double salt CsCe(SeO4)2·4H2O can be obtained from mixing cerium(III) selenate and cesium selenate in an aqueous solution, and then evaporating and crystallizing the solution.[3]
References
- ↑ 化学化工物性数据手册(无机卷).刘光启 等主编.化学工业出版社.16.2 硒酸盐.P569
- ↑ Wickleder, Mathias S. (2005), "Oxo-Selenates of rare earth elements", Handbook on the Physics and Chemistry of Rare Earths (Elsevier): pp. 45–105, http://dx.doi.org/10.1016/s0168-1273(05)35002-1, retrieved 2023-12-05
- ↑ OVANESYAN, S. M.; ISKHAKOVA, L. D.; TRUNOV, V. K. (1987-06-30). "ChemInform Abstract: Synthesis and Crystal Structure of CsLn(SeO4)2·4 H2O.". ChemInform 18 (26). doi:10.1002/chin.198726005. ISSN 0931-7597. http://dx.doi.org/10.1002/chin.198726005.
Template:SelenatesTemplate:Inorganic-compound-stubcategory:Selenates
 |

