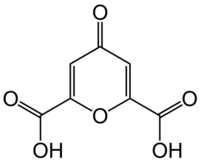
Chemistry:Chelidonic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Oxo-4H-pyran-2,6-dicarboxylic acid | |
| Other names
Jerva acid; Jervaic acid; Jervasic acid; γ-Pyrone-2,6-dicarboxylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H4O6 | |
| Molar mass | 184.103 g·mol−1 |
| Melting point | 257 °C (495 °F; 530 K)[1] (decomposes) |
| Related compounds | |
Related compounds
|
Meconic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Chelidonic acid is a heterocyclic organic acid with a pyran skeleton.
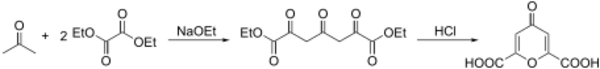
Preparation
Chelidonic acid can be prepared in two steps from diethyl oxalate and acetone:[1][2]
Uses
Chelidonic acid is used to synthesize 4-pyrone via thermal decarboxylation.[3]
Natural occurrence
Chelidonic acid was first discovered in extracts of Chelidonium majus.[4][5][6] It occurs naturally in plants of the Asparagales order.[7] Potassium chelidonate has been found to be responsible for nyctinasty in some plants; specifically, it has been found to regulate the closing of leaves of Cassia mimosoides at nightfall.[8][9]
See also
References
- ↑ 1.0 1.1 E. Raymond Riegel and F. Zwilgmeyer (1937). "Chelidonic acid". Organic Syntheses 17: 40. http://www.orgsyn.org/demo.aspx?prep=CV2P0126.; Collective Volume, 2, pp. 126
- ↑ G. Horvath; C. Russa; Z. Koentoes; J. Gerencser (1999). "A new Efficient Method for the Preparation of 2,6-Pyridinedihiethyl Ditosylates from Dimethyl 2,60-Pyridinedicarboxylates". Synth. Commun. 29 (21): 3719–3732. doi:10.1080/00397919908086011.
- ↑ Weygand, Conrad (1972). Hilgetag, G.; Martini, A.. eds. Weygand/Hilgetag Preparative Organic Chemistry (4th ed.). New York: John Wiley & Sons, Inc.. p. 1009. ISBN 0471937495.
- ↑ Roscoe, H.E.; Schorlemmer, C. (1890). A Treatise on Chemistry, Volume 3, Part 2 (1st ed.). New York: D Appleton and Company. pp. 624. https://books.google.com/books?id=xM4cAQAAIAAJ&pg=PA624.
- ↑ Probst, Joseph M. A. (1839) "Beschreibung und Darstellungsweise einiger bei der Analyse des Chelidonium majus aufgefundenen Stoffe" (Description and methods of preparation of some substances found during the analysis of Chelidonium majus), Annalen der Chemie und Pharmacie, 29 (2) : 113–131 ; see especially pp. 116–118.
- ↑ See also: Lerch, Joseph Udo (1846) "Untersuchung der Chelidonsäure" (Investigation of chelidonic acid), Annalen der Chemie und Pharmacie, 57 : 273–318.
- ↑ "Asparagales". Angiosperm Phylogeny Group. http://www.mobot.org/MOBOT/research/APweb/orders/asparagalesweb.htm#Asparagales. Retrieved 30 August 2017.
- ↑ Ueda, Minoru; Ohnuki, Takashi; Yamamura, Shosuke (1998). "Leaf-opening substance of a nyctinastic plant, Cassia mimosoides". Phytochemistry 49 (3): 633. doi:10.1016/S0031-9422(98)00134-4.
- ↑ Ueda, Minoru; Yamamura, Shosuke (1998). "Chemical studies on plant movement". Current Organic Chemistry 2 (4): 437–461. https://books.google.com/books?id=aI_3BDcYKXgC&pg=PA437.
 |