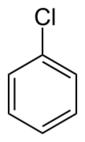
Chemistry:Chlorobenzene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chlorobenzene | |||
| Other names
Phenyl chloride, monochlorobenzene[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| Abbreviations | PhCl | ||
| 605632 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 26704 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1134 | ||
| |||
| |||
| Properties | |||
| C6H5Cl | |||
| Molar mass | 112.56 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | almond-like[2] | ||
| Density | 1.11 g/cm3, liquid | ||
| Melting point | −45.58 °C (−50.04 °F; 227.57 K) | ||
| Boiling point | 131.70 °C (269.06 °F; 404.85 K) | ||
| 0.5 g l−1 in water at 20 °C | |||
| Solubility in other solvents | soluble in most organic solvents | ||
| Vapor pressure | 9 mmHg[2] | ||
| −69.97·10−6 cm3/mol | |||
Refractive index (nD)
|
1.52138 | ||
| Viscosity | 0.7232 | ||
| Hazards | |||
| Main hazards | Low to moderate hazard[3] | ||
| GHS pictograms |   
| ||
| GHS Signal word | Warning | ||
| H226, H302, H305, H315, H332, H411 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P302+352, P303+361+353, P304+312, P304+340, P312, P321, P332+313, P362, P370+378, P391, P403+235, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 29 °C (84 °F; 302 K) | ||
| Explosive limits | 1.3%-9.6%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
2290 mg/kg (rat, oral)
590 mg/kg (mouse, orally)[4] | ||
LCLo (lowest published)
|
8000 ppm (cat, 3 hr)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 75 ppm (350 mg/m3)[2] | ||
REL (Recommended)
|
none[2] | ||
IDLH (Immediate danger)
|
1000 ppm[2] | ||
| Related compounds | |||
Related Halobenzenes
|
Fluorobenzene Bromobenzene Iodobenzene | ||
Related compounds
|
benzene 1,4-dichlorobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chlorobenzene is the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.[6]
Uses
Chlorobenzene is used as a high-boiling solvent in industrial applications as well as in the laboratory.[7]
Chlorobenzene is nitrated on a large scale to give a mixture of 2-nitrochlorobenzene and 4-nitrochlorobenzene, which are separated. These mononitrochlorobenzenes are converted to related 2-nitrophenol, 2-nitroanisole, bis(2-nitrophenyl)disulfide, and 2-nitroaniline by nucleophilic displacement of the chloride, with respectively sodium hydroxide, sodium methoxide, sodium disulfide, and ammonia. The conversions of the 4-nitro derivative are similar.[8]
Historical
The major use of chlorobenzene is as an intermediate in the production of herbicides, dyestuffs, and rubber.
Chlorobenzene once was used in the manufacture of pesticides, most notably DDT, by reaction with chloral (trichloroacetaldehyde), but this application has declined with the diminished use of DDT. At one time, chlorobenzene was the main precursor for the manufacture of phenol:[9]
- C6H5Cl + NaOH → C6H5OH + NaCl
The reaction also has a byproduct of salt. The reaction is known as the Dow process, with the reaction carried out at 350 °C using fused sodium hydroxide without solvent. Labeling experiments show that the reaction proceeds via elimination/addition, through benzyne as the intermediate.
Production
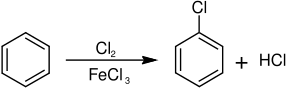
It was first described in 1851. Chlorobenzene is manufactured by chlorination of benzene in the presence of a catalytic amount of Lewis acid such as ferric chloride, sulfur dichloride, and anhydrous aluminium chloride:[6]
The catalyst enhances the electrophilicity of the chlorine. Because chlorine is electronegative, C6H5Cl exhibits somewhat decreased susceptibility to further chlorination. Industrially the reaction is conducted as a continuous process to minimize the formation of dichlorobenzenes.
Laboratory routes
Chlorobenzene is producible from aniline via benzenediazonium chloride, otherwise known as the Sandmeyer reaction.
Safety
Chlorobenzene exhibits "low to moderate" toxicity as indicated by its -1">50 of 2.9 g/kg.[7] The Occupational Safety and Health Administration has set a permissible exposure limit at 75 ppm (350 mg/m3) over an eight-hour time-weighted average for workers handling chlorobenzene.[10]
Toxicology and biodegradation
Chlorobenzene can persist in soil for several months, in air for about 3.5 days, and in water for less than one day. Humans may be exposed to this agent via breathing contaminated air (primarily via occupational exposure), consuming contaminated food or water, or by coming into contact with contaminated soil (typically near hazardous waste sites). However, because it has only been found at 97 out of 1,177 NPL hazardous waste sites, it is not considered a widespread environmental contaminant. The bacterium Rhodococcus phenolicus degrades chlorobenzene, dichlorobenzene and phenol as sole carbon sources.[11]
Upon entering the body, typically via contaminated air, chlorobenzene is excreted both via the lungs and the urinary system.
On other planets
Chlorobenzene has been detected in a sedimentary rock on Mars.[12] It was speculated that the chlorobenzene might have been produced when the sample was heated in the instrument sampling chamber. The heating would have triggered a reaction of organics in the Martian soil, which is known to contain perchlorate.
References
- ↑ Pubchem. "Chlorobenzene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/Chlorobenzene#section=Synonyms.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 NIOSH Pocket Guide to Chemical Hazards. "#0121". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0121.html.
- ↑ Chlorobenzene toxicity
- ↑ Chlorobenzene: LD50
- ↑ 5.0 5.1 "Chlorobenzene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/108907.html.
- ↑ 6.0 6.1 U. Beck, E. Löser "Chlorinated Benzenes and other Nucleus-Chlorinated Aromatic Hydrocarbons" Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o06_o03
- ↑ 7.0 7.1 Rossberg, Manfred; Lendle, Wilhelm; Pfleiderer, Gerhard; Tögel, Adolf; Dreher, Eberhard-Ludwig; Langer, Ernst; Rassaerts, Heinz; Kleinschmidt, Peter et al. (2006). "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a06_233.pub2. ISBN 3527306730.
- ↑ Gerald Booth (2007). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- ↑ Weber, Manfred; Weber, Markus; Kleine-Boymann, Michael (2004). "Phenol". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_299.pub2. ISBN 3527306730.
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
- ↑ Rehfuss, M.; Urban, J. (2005). "Rhodococcus phenolicus sp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources". Systematic and Applied Microbiology 28 (8): 695–701. doi:10.1016/j.syapm.2005.05.011. PMID 16261859. Erratum: Rehfuss, M. (2006). "Erratum to "Rhodococcus phenolicus sp. nov., a novel bioprocessor isolated actinomycete with the ability to degrade chlorobenzene, dichlorobenzene and phenol as sole carbon sources" [Systematic and Applied Microbiology 28 (2005) 695–701]". Systematic and Applied Microbiology 29 (2): 182. doi:10.1016/j.syapm.2005.11.005.
- ↑ Freissinet, C. (2015). "Organic molecules in the sheepbed mudstone, gale crater, mars". Journal of Geophysical Research: Planets 120 (3): 495–514. doi:10.1002/2014JE004737. PMID 26690960. Bibcode: 2015JGRE..120..495F.
External links
 |