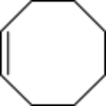
Chemistry:Cis-Cyclooctene
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Z)-Cyclooctene | |
| Other names
cis-Cyclooctene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H14 | |
| Molar mass | 110.200 g·mol−1 |
| Density | 0.846 g/mL |
| Melting point | −16 °C (3 °F; 257 K) |
| Boiling point | 145 to 146 °C (293 to 295 °F; 418 to 419 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H226, H304 | |
| P210, P233, P240, P241, P242, P243, P280, P301+310, P303+361+353, P331, P370+378, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
cis-Cyclooctene is a cycloalkene with the formula (CH2)6(CH)2. It is a colorless liquid that is used industrially to produce a polymer. It is also a ligand in organometallic chemistry.
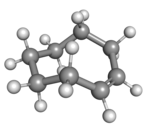
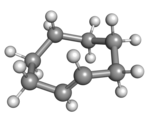
Cyclooctene is the smallest cycloalkene that can be isolated as both the cis- and trans-isomer.[2] cis-Cyclooctene is shaped like the 8-carbon equivalent chair conformation of cyclohexane.
 |
|
| cis-Cyclooctene in chair conformation |
(Rp)-trans-Cyclooctene in crown conformation |
Uses and reactions
Cyclooctene undergoes ring-opening metathesis polymerization to give polyoctenamers, which are marketed under the name Vestenamer.[3]
cis-Cyclooctene (COE) is a substrate known for quite selectively forming the epoxide, as compared to other cycloalkenes, e.g. cyclohexene. Low amounts of radical by-products are found only. This behaviour is attributed to the difficulty of functionalizing allylic CH centers, which almost orthogonal allylic C-H bonds. Therefore, if radicals are around, they tend to form epoxide via an addition-elimination mechanism.[2]
It is used as an easily displaced ligand in organometallic chemistry, e.g. chlorobis(cyclooctene)rhodium dimer and chlorobis(cyclooctene)iridium dimer.
References
- ↑ "cis-Cyclooctene". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/125482.
- ↑ 2.0 2.1 Neuenschwander, Ulrich; Hermans, Ive (2011). "The Conformations of Cyclooctene: Consequences for Epoxidation Chemistry". J. Org. Chem. 76 (24): 10236–10240. doi:10.1021/jo202176j. PMID 22077196.
- ↑ Lionel Delaude; Alfred F. Noels (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 0471238961.
 |

