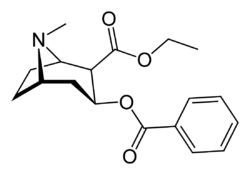
Chemistry:Cocaethylene
 | |
 | |
| Clinical data | |
|---|---|
| Other names | benzoylecgonine ethyl ester, ethylbenzoylecgonine, |
| Pregnancy category |
|
| Routes of administration | Produced from ingestion of cocaine and ethanol |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H23NO4 |
| Molar mass | 317.385 g·mol−1 |
| 3D model (JSmol) | |
| |
Cocaethylene (ethylbenzoylecgonine) is the ethyl ester of benzoylecgonine. It is structurally similar to cocaine, which is the methyl ester of benzoylecgonine. Cocaethylene is formed by the liver when cocaine and ethanol coexist in the blood.[1] In 1885, cocaethylene was first synthesized (according to edition 13 of the Merck Index),[2] and in 1979, cocaethylene's side effects were discovered.[3]
Metabolic production from cocaine
Cocaethylene is the byproduct of concurrent consumption of alcohol and cocaine as metabolized by the liver. Normally, metabolism of cocaine produces two primarily biologically inactive metabolites—benzoylecgonine and ecgonine methyl ester. The hepatic enzyme carboxylesterase is an important part of cocaine's metabolism because it acts as a catalyst for the hydrolysis of cocaine in the liver, which produces these inactive metabolites. If ethanol is present during the metabolism of cocaine, a portion of the cocaine undergoes transesterification with ethanol, rather than undergoing hydrolysis with water, which results in the production of cocaethylene.[1]
- cocaine + H2O → benzoylecgonine + methanol (with liver carboxylesterase 1)[4]
- benzoylecgonine + ethanol → cocaethylene + H2O
- cocaine + ethanol → cocaethylene + methanol (with liver carboxylesterase 1)[5]
Physiological effects
Cocaethylene is largely considered a recreational drug in and of itself, with stimulant, euphoriant, anorectic, sympathomimetic, and local anesthetic properties. The monoamine neurotransmitters serotonin, norepinephrine, and dopamine play important roles in cocaethylene's action in the brain. Cocaethylene increases the levels of serotonergic, noradrenergic, and dopaminergic neurotransmission in the brain by inhibiting the action of the serotonin transporter, norepinephrine transporter, and dopamine transporter. These pharmacological properties make cocaethylene a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI; also known as a "triple reuptake inhibitor").[citation needed]
In most users, cocaethylene produces euphoria and has a longer duration of action than cocaine.[6][7] Some studies[8][9] suggest that consuming alcohol in combination with cocaine may be more cardiotoxic than cocaine and "it also carries an 18 to 25 fold increase over cocaine alone in risk of immediate death".[7] Cocaethylene has a higher affinity for the dopamine transporter than does cocaine, but has a lower affinity for the serotonin and norepinephrine transporters.[10][11]
In McCance-Katz et alia's 1993 study found that cocaethylene "produced greater subjective ratings of 'High' in comparison with administration of cocaine or alcohol alone."[6]
See also
- Ethylphenidate
- Euphoriants
- Methylvanillylecgonine
- Local anesthetics
- Stimulants
- Tropanes
- Vin Mariani
- Pemberton's French Wine Coca
References
- ↑ 1.0 1.1 "Cocaethylene metabolism and interaction with cocaine and ethanol: role of carboxylesterases". Drug Metabolism and Disposition 31 (1): 16–20. January 2003. doi:10.1124/dmd.31.1.16. PMID 12485948.
- ↑ "Forensic Drug Profile: Cocaethylene". Journal of Analytical Toxicology 43 (3): 155–160. April 2019. doi:10.1093/jat/bkz007. PMID 30796807.
- ↑ * "Warning of extra heart dangers from mixing cocaine and alcohol". The Guardian. 8 November 2009. https://www.theguardian.com/society/2009/nov/08/cocaine-alcohol-mixture-health-risks.
- ↑ "MetaCyc Reaction: 3.1.1.". http://biocyc.org/META/NEW-IMAGE?type=REACTION&object=RXN-13424.
- ↑ "MetaCyc Reaction: [no EC number assigned"]. http://biocyc.org/META/NEW-IMAGE?type=REACTION&object=RXN-13425.
- ↑ 6.0 6.1 "Comparison of intravenous cocaethylene and cocaine in humans". Psychopharmacology 149 (2): 153–162. April 2000. doi:10.1007/s002139900363. PMID 10805610.
- ↑ 7.0 7.1 "Cocaethylene toxicity". Journal of Addictive Diseases 16 (3): 75–84. 1997. doi:10.1300/J069v16n03_08. PMID 9243342.
- ↑ "Cocaine, ethanol, and cocaethylene cardiotoxity in an animal model of cocaine and ethanol abuse". Academic Emergency Medicine 8 (3): 211–222. March 2001. doi:10.1111/j.1553-2712.2001.tb01296.x. PMID 11229942.
- ↑ "Alcohol and cocaine interactions in humans". The Journal of Pharmacology and Experimental Therapeutics 266 (3): 1364–1373. September 1993. PMID 8371143.
- ↑ "Alcohol plus cocaine: the whole is more than the sum of its parts". Therapeutic Drug Monitoring 18 (4): 460–464. August 1996. doi:10.1097/00007691-199608000-00026. PMID 8857569.
- ↑ "Comparison in humans of the potency and pharmacokinetics of intravenously injected cocaethylene and cocaine". Psychopharmacology 116 (4): 428–432. December 1994. doi:10.1007/bf02247473. PMID 7701044.
Further reading
- "Cocaethylene: responding to combined alcohol and cocaine use". Alcohol Policy UK. 19 April 2010. http://www.alcoholpolicy.net/2010/04/cocaethylene-responding-to-combined-alcohol-and-cocaine-use.html.
- "An overview of cocaethylene, an alcohol-derived, psychoactive, cocaine metabolite". Journal of Psychoactive Drugs 24 (3): 273–276. 1992. doi:10.1080/02791072.1992.10471648. PMID 1432406.
- "Cocaethylene is more potent than cocaine in mediating lethality". Pharmacology, Biochemistry, and Behavior 39 (2): 531–533. June 1991. doi:10.1016/0091-3057(91)90222-N. PMID 1946594.
- "Cocaethylene: a unique cocaine metabolite displays high affinity for the dopamine transporter". Journal of Neurochemistry 56 (2): 698–701. February 1991. doi:10.1111/j.1471-4159.1991.tb08205.x. PMID 1988563.
 |
